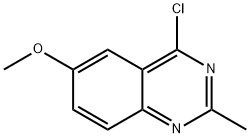
4-Chloro-6-methoxy-2-methylquinazoline synthesis
- Product Name:4-Chloro-6-methoxy-2-methylquinazoline
- CAS Number:60395-90-6
- Molecular formula:C10H9ClN2O
- Molecular Weight:208.64
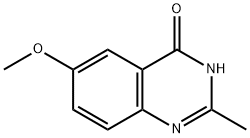
51413-71-9

60395-90-6
6-Methoxy-2-methylquinazolin-4-ol (233 mg, 1.23 mmol) was used as starting material and suspended in phosphoryl chloride (POCl3, 10 mL, 108 mmol). The reaction mixture was stirred under reflux conditions for 2.5 days and a gradual transformation of the suspension into a reddish brown solution was observed. Upon completion of the reaction, the mixture was cooled to room temperature and the excess phosphoryl chloride was subsequently removed by evaporation under reduced pressure. The residue was partitioned between 5% aqueous sodium bicarbonate solution (NaHCO3, 100 mL) and ethyl acetate (100 mL) for extraction. The organic layer was washed sequentially with 5% aqueous sodium bicarbonate solution (100 mL) and pure water (100 mL). The washed organic layer was dried with anhydrous sodium sulfate (Na2SO4) and subsequently concentrated under reduced pressure. The crude product was purified by silica gel column chromatography using cyclohexane/ethyl acetate as eluent. The final target product, 4-chloro-6-methoxy-2-methylquinazoline, was obtained as a white solid (142 mg, 0.681 mmol) in 56% yield. The product was characterized by 1H NMR (400 MHz, DMSO-d6) and UPLC-MS (ESI): 1H NMR δ 7.92 (d, J = 9.1 Hz, 1H), 7.70 (dd, J = 9.1, 2.8 Hz, 1H), 7.42 (d, J = 2.8 Hz, 1H), 3.96 (s, 3H), 2.71 (s, 3H); UPLC-MS (ESI) RT 3.88 min, m/z 209 [M + H]+ (purity >95%).

51413-71-9
24 suppliers
inquiry

60395-90-6
33 suppliers
$45.00/2.5mg
Yield:60395-90-6 56%
Reaction Conditions:
with trichlorophosphate for 60 h;Reflux;
Steps:
17 4.1.2.17
4-Chloro-6-methoxy-2-methylquinazoline (22)
6-methoxy-2-methylquinazolin-4-ol (233 mg, 1.23 mmol) was suspended in phosphorus oxychloride (POCl3) (10 mL, 108 mmol) and the reaction mixture was stirred under reflux for 2.5 days during which the suspension turned into a reddish brown solution.
The mixture was allowed to cool down to room temperature and phosphorus oxychloride was evaporated under reduced pressure.
Then, the residue was partitioned between aqueous solution of sodium bicarbonate (NaHCO3, 5%, 100 mL) and ethyl acetate (100 mL). The organic layers was washed with aqueous solution of sodium bicarbonate (NaHCO3, 5%, 100 mL) and water (100 mL). Then, the organic layer was dried over sodium sulfate (Na2SO4) and evaporated under reduced pressure.
The residue was purified by silica gel column chromatography using cyclohexane/ethyl acetate as eluent. Yield 56% (white solid, 142 mg, 0681 mmol); 1H NMR (400 MHz, DMSO-d6) δ ppm 7.92 (d, J = 9.1 Hz, 1H), 7.70 (dd, J = 9.1, 2.8 Hz, 1H), 7.42 (d, J = 2.8 Hz, 1H), 3.96 (s, 3H), 2.71 (s, 3H); UPLC-MS (ESI) (B); RT 3.88 min, m/z 209 [M+H]+ (>95%).
References:
Pitta, Eleni;Balabon, Olga;Rogacki, Maciej K.;Gómez, Jesús;Cunningham, Fraser;Joosens, Jurgen;Augustyns, Koen;van der Veken, Pieter;Bates, Robert [European Journal of Medicinal Chemistry,2017,vol. 125,p. 890 - 901]
6705-03-9
275 suppliers
$9.00/1g

60395-90-6
33 suppliers
$45.00/2.5mg