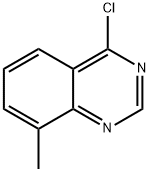
4-CHLORO-8-METHYLQUINAZOLINE synthesis
- Product Name:4-CHLORO-8-METHYLQUINAZOLINE
- CAS Number:58421-80-0
- Molecular formula:C9H7ClN2
- Molecular Weight:178.62
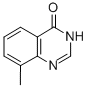
19181-54-5

58421-80-0
General procedure for the synthesis of 4-chloro-8-methylquinazoline from 8-methyl-4-quinazolone: Phosphorus oxychloride (800 mL) was added to a 2 L round-bottomed flask under nitrogen protection. Subsequently, 8-methylquinazolin-4(3H)-one (125 g) was added in batches. The reaction mixture was heated to 120 °C and refluxed for 12 hours. The reaction process was monitored by thin layer chromatography (TLC) and liquid chromatography-mass spectrometry (LCMS). Upon completion of the reaction, the mixture was cooled to room temperature and evaporated to dryness under reduced pressure. The resulting residue was dissolved in dichloromethane (DCM, 500 mL) and quenched by slow pouring into a pre-cooled saturated potassium carbonate (K2CO3) solution under continuous stirring. The organic layer was separated, washed with brine, dried over anhydrous sodium sulfate, and subsequently concentrated under vacuum to afford 4-chloro-8-methylquinazoline (120 g, 86% yield) as a yellow solid. The product could be used for subsequent reactions without further purification. Mass spectrometry (MS) data: m/z = 179/181 [M + H]+.

19181-54-5
82 suppliers
$21.00/250mg

58421-80-0
82 suppliers
$23.00/250mg
Yield:58421-80-0 86%
Reaction Conditions:
with trichlorophosphate at 120; for 12 h;Inert atmosphere;
Steps:
4-Chloro-8-methylquinazoline:
Phosphorous oxychloride (800 mL) was taken in a 2 L round bottom flask under nitrogen. To this was added 8-Methylquinazolin-4(3H)-one (125 g) in portions. The reactionminxture refluxed at 120 °C for 12h. Reaction completion was monitored by TLC and LCMS. After completion, the reactionminxture was cooled to RT and evaporated to dryness under reduced pressure. The resulted residue was dissolved in DCM (500 mL) and quenched slowly into an ice cold solution of saturated K2C03 with constant stirring. Then the organic layer was separated and washed with brine solution, dried over sodium sulfate and concentrated under vacuum to afford 4-chloro-8-methylquinazoline (120 g, 86%) as yellow solid. This was taken for next step without further purification. MS: m/z = 179/18 1 [M+Hj.
References:
MERCK PATENT GMBH;SHERER, Brian A.;BRUGGER, Nadia WO2017/106607, 2017, A1 Location in patent:Paragraph 00224
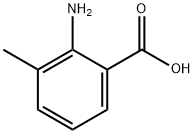
4389-45-1
504 suppliers
$5.00/5g

58421-80-0
82 suppliers
$23.00/250mg
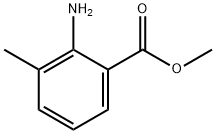
22223-49-0
241 suppliers
$5.00/1g

58421-80-0
82 suppliers
$23.00/250mg