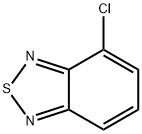
4-Chlorobenzo[c][1,2,5]thiadiazole synthesis
- Product Name:4-Chlorobenzo[c][1,2,5]thiadiazole
- CAS Number:2207-28-5
- Molecular formula:C6H3ClN2S
- Molecular Weight:170.62
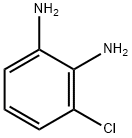
21745-41-5
98 suppliers
$42.00/1g
![4-Chlorobenzo[c][1,2,5]thiadiazole](/CAS/20150408/GIF/2207-28-5.gif)
2207-28-5
31 suppliers
inquiry
Yield:2207-28-5 93%
Reaction Conditions:
with N-phenylsulfinylamine in toluene at 150; for 5 h;Inert atmosphere;
Steps:
4.1.1. Synthesis of 4-chlorobenzo[c] [1,2,5]thiadiazole (9a)
N-Thionylaniline (700 mg, 5 mmol), 8 (142 mg, 1 mmol) andtoluene (5 mL) was added into a flask. The mixture was stirred at150 C for 5 h. After the completion of the reaction as indicated by TLC, the mixture was concentrated under a reduced pressure. H2O(10 mL) was added to the residue and the resulting mixture wasextracted with EtOAc. The combined organic layer was dried overNa2SO4, concentrated under a reduced pressure to give 9a as a graysolid (180 mg, 93%).1H NMR (400 MHz, CDCl3) d 7.93 (d, J 8.7 Hz,1H), 7.64 (d, J 7.2 Hz, 1H), 7.54 (dd, J 8.8, 7.2 Hz, 1H).
References:
Ding, Chunyong;He, Yinyan;Li, Peng;Li, Yongling;Liu, Jingting;Ma, Jing;Qian, Feng;Sun, Zhen-Liang;Wang, Linlin;Xiao, Ruoxuan;Zhang, Ao;Zhao, Jiannan [European Journal of Medicinal Chemistry,2022,vol. 237,art. no. 114338]
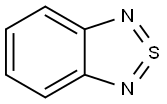
22706-22-5
15 suppliers
inquiry
![4-Chlorobenzo[c][1,2,5]thiadiazole](/CAS/20150408/GIF/2207-28-5.gif)
2207-28-5
31 suppliers
inquiry