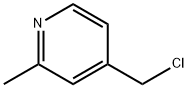
4-(CHLOROMETHYL)-2-METHYLPYRIDINE synthesis
- Product Name:4-(CHLOROMETHYL)-2-METHYLPYRIDINE
- CAS Number:75523-42-1
- Molecular formula:C7H8ClN
- Molecular Weight:141.6
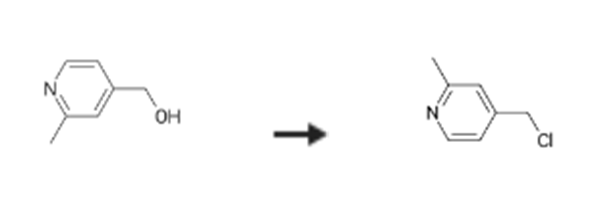
5-[(2-Chlorophenyl)acetylamino]-3-(4-fluorophenyl)-4-[4-(2-methylpyridyl)]isoxazole a) 4-Chloromethyl-2-methylpyridine; Into 100 mL of methylene chloride solution containing 2. 1-6 g of 4-(2-methylpyridyl)methanol (cf. PCT International Publication WO98/2120 Pamphlet), 23 mL of thionyl chloride was dropped at room temperature, followed by 20 hours' stirring at room temperature. The solvent was distilled off from the reaction solution under reduced pressure, and the residue was extracted with methylene chloride, after addition of saturated aqueous NaHCO3 solution. The methylene chloride extract was dried over anhydrous magnesium sulfate, and from which the solvent was distilled off under reduced pressure to provide 2.46 g (yield: 100%) of brown crystalline title compound. 1H-NMR(CDCl3)delta:8.45(d,J=5.0Hz,1H),7.31(s,1H), 7.25(d,J=5.0Hz,1H),4.74(s,2H),2.48(s,3H) Mass,m/e:141 (M+,base).
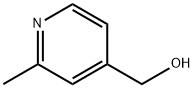
105250-16-6
112 suppliers
$35.00/100mg

75523-42-1
31 suppliers
$1320.00/5g
Yield:75523-42-1 100%
Reaction Conditions:
Stage #1: (2-methylpyridin-4-yl)methanolwith thionyl chloride in dichloromethane at 20; for 20 h;
Stage #2: with water;sodium hydrogencarbonate
Steps:
196.a
Example 196 5-[(2-Chlorophenyl)acetylamino]-3-(4-fluorophenyl)-4-[4-(2-methylpyridyl)]isoxazole a) 4-Chloromethyl-2-methylpyridine; Into 100 mL of methylene chloride solution containing 2. 1-6 g of 4-(2-methylpyridyl)methanol (cf. PCT International Publication WO98/2120 Pamphlet), 23 mL of thionyl chloride was dropped at room temperature, followed by 20 hours' stirring at room temperature. The solvent was distilled off from the reaction solution under reduced pressure, and the residue was extracted with methylene chloride, after addition of saturated aqueous NaHCO3 solution. The methylene chloride extract was dried over anhydrous magnesium sulfate, and from which the solvent was distilled off under reduced pressure to provide 2.46 g (yield: 100%) of brown crystalline title compound. 1H-NMR(CDCl3)δ:8.45(d,J=5.0Hz,1H),7.31(s,1H), 7.25(d,J=5.0Hz,1H),4.74(s,2H),2.48(s,3H) Mass,m/e:141 (M+,base)
References:
EP2036905,2009,A1 Location in patent:Page/Page column 49
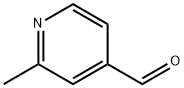
63875-01-4
144 suppliers
$32.00/100mg

75523-42-1
31 suppliers
$1320.00/5g
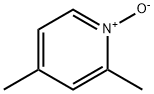
1122-45-8
34 suppliers
inquiry

75523-42-1
31 suppliers
$1320.00/5g