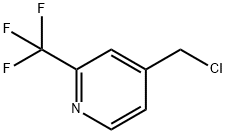
4-ChloroMethyl-2-trifluoroMethyl-pyridine synthesis
- Product Name:4-ChloroMethyl-2-trifluoroMethyl-pyridine
- CAS Number:1027545-48-7
- Molecular formula:C7H5ClF3N
- Molecular Weight:195.57
131747-61-0
94 suppliers
$100.00/100mg

1027545-48-7
35 suppliers
inquiry
Yield:1027545-48-7 60%
Reaction Conditions:
with thionyl chloride in dichloromethane at 0 - 60; for 1 h;
Steps:
2 Step 2: 4-(chloromethyl)-2-(trifluoromethyl)pyridine
To a mixture of (2- (trifluoromethyl) pyridin-4-yl) methanol A-2 (400 mg, 2 mmol) in dichloromethane (5mL) was added SOCl2(2mL) slowly at 0 . The reaction mixture wa stirred at 60 for 1 h. The reaction mixture was then quenched with 1 mL saturated aqueous NH4Cl and extracted with ethyl acetate (5mL × 3) . The combined organic extracts were concentrated in vacuo, the residue was purified on silica gel (EtOAc: petroleum ether 1: 5) to give the product as a solid (300 mg, 60 ) MS (ESI) calc’dfor (C7H5ClF3N) [M+H]+: 195.0 found: 195
References:
WO2016/29454,2016,A1 Location in patent:Page/Page column 59; 60
131747-41-6
210 suppliers
$10.00/1g

1027545-48-7
35 suppliers
inquiry