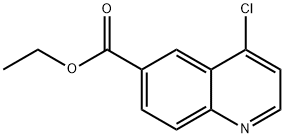
4-Chloroquinoline-6-carboxylic acid ethyl ester synthesis
- Product Name:4-Chloroquinoline-6-carboxylic acid ethyl ester
- CAS Number:148018-34-2
- Molecular formula:C12H10ClNO2
- Molecular Weight:235.67

127286-04-8

148018-34-2
The general procedure for the synthesis of ethyl 4-chloroquinoline-6-carboxylate from the compound (CAS:127286-04-8) was as follows: intermediate 41 (3 g, 13.8 mmol) was mixed with phosphorus trichloride (8 mL) and the reaction was stirred at 120 °C for 3 hours. Upon completion of the reaction, the solvent was removed by vacuum evaporation and subsequently quenched by adding crushed ice to the residue. Next, saturated aqueous sodium bicarbonate solution with ethyl acetate was added to the mixture to form a two-phase system. The organic and aqueous phases were separated and the aqueous phase was then extracted with ethyl acetate. All organic phases were combined, dried with anhydrous sodium sulfate, filtered to remove the desiccant, and the filtrate was evaporated under vacuum to remove the volatile components. Finally, the target product ethyl 4-chloroquinoline-6-carboxylate (2.19 g, 67% yield) was purified by silica gel column chromatography.
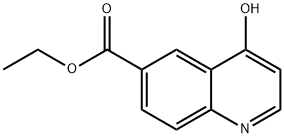
148018-33-1
41 suppliers
$65.00/50mg

148018-34-2
45 suppliers
$70.00/1g
Yield:148018-34-2 67%
Reaction Conditions:
with trichlorophosphate at 120; for 3 h;
Steps:
10.42
Intermediate 41 (3 g 13.8 mmol) and POCl2 (8 mL) were combined and stirred for 3 h at 120° C. The solvent was evaporated in vacuo and crushed ice was added. A biphasic mixture of Saturated aqueous NaHCO3 and ethyl acetate was added. The layers were separated and the aqueous phase was extracted with ethyl acetate. The organic phases were combined, dried over solid Na2SO4, filtered, and the volatiles from the filtrate were evaporated in vacuo. The desired product was purified by Silica gel column chromatography (2.19 g, 67%).
References:
US2008/234267,2008,A1 Location in patent:Page/Page column 25