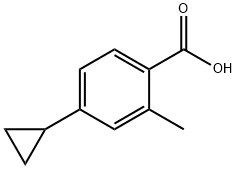
4-cyclopropyl-2-methylbenzoic acid synthesis
- Product Name:4-cyclopropyl-2-methylbenzoic acid
- CAS Number:909698-10-8
- Molecular formula:C11H12O2
- Molecular Weight:176.21
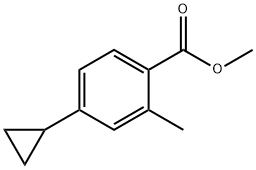
909698-09-5

909698-10-8
To a stirred mixed solution of methyl 4-cyclopropyl-2-methylbenzoate (1.3 g, 6.84 mmol) in tetrahydrofuran (THF, 9 mL), methanol (MeOH, 6 mL) and water (3 mL) was added lithium hydroxide monohydrate (0.842 g, 20.52 mmol). The reaction mixture was stirred at 70 °C for 2 hours. After completion of the reaction, excess solvent was removed by distillation under reduced pressure. The residue was dissolved in water and acidified to pH < 7 with 10% hydrochloric acid (HCl) solution, followed by three extractions with ethyl acetate (EtOAc). The organic phases were combined, dried with anhydrous sodium sulfate, filtered and concentrated under reduced pressure to afford the target product 4-cyclopropyl-2-methylbenzoic acid (1.2 g, 99% yield). The product was characterized by 1H NMR (400 MHz, DMSO-d6): δ 7.52 (d, J = 7.6 Hz, 1H), 6.75-6.74 (m, 2H), 1.84-1.81 (m, 1H), 0.92-0.87 (m, 2H), 0.64-0.60 (m, 2H).

909698-09-5
8 suppliers
inquiry

909698-10-8
38 suppliers
$22.00/100mg
Yield:909698-10-8 99%
Reaction Conditions:
with lithium hydroxide monohydrate;water in tetrahydrofuran;methanol at 70; for 2 h;
Steps:
Step-c: Synthesis of 4-cyclopropyl-2-methylbenzoic acid
To a stirred solution of methyl 4-cyclopropyl-2-methylbenzoate (1.3 g, 6.84 mmol) in THF (9 mL), MeOH (6 mL) and water (3 ml) was added lithium hydroxide monohydrate (0.842 g, 20.52 mmol). The reaction mixture was stirred at 70°C for 2 h, excess solvent was removed under reduced pressure. The aqueous layer was acidified with 10 % HC1 solution and then extracted with EtOAc. The organic extracts were dried and concentrated to afford the title compound (1.2 g, 99 %). *H NMR (400 MHz, DMSO-d6): δ 7.52 (d, J=7.6 Hz, 1H), 6.75-6.74 (m, 2H), 1.84-1.81 (m, 1H), 0.92-0.87 (m, 2H), 0.64-0.60 (m, 2H).
References:
WO2014/125408,2014,A2 Location in patent:Page/Page column 24
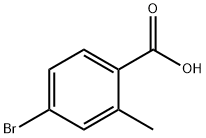
68837-59-2
349 suppliers
$5.00/1g

909698-10-8
38 suppliers
$22.00/100mg
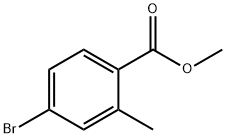
99548-55-7
173 suppliers
$6.00/1g

909698-10-8
38 suppliers
$22.00/100mg