
4-ETHOXY-2-FLUOROBENZALDEHYDE synthesis
- Product Name:4-ETHOXY-2-FLUOROBENZALDEHYDE
- CAS Number:532965-74-5
- Molecular formula:C9H9FO2
- Molecular Weight:168.16
348-27-6

75-03-6

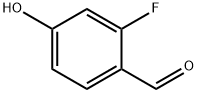
532965-74-5
At 25 °C and under nitrogen protection, 2-fluoro-4-hydroxybenzaldehyde (1.0 g, 7.1 mmol) and potassium carbonate (2.0 g, 14 mmol) were dissolved in N,N-dimethylformamide (10 mL), followed by the addition of ethyl iodide (1.1 g, 7.1 mmol). The reaction mixture was stirred at 60 °C for 10 hours. After completion of the reaction, the mixture was concentrated in vacuum, diluted with ethyl acetate (200 mL), washed sequentially with saturated sodium bicarbonate solution (3 x 50 mL) and brine (3 x 50 mL), and the organic phase was dried with anhydrous sodium sulfate. After drying, the organic phase was concentrated in vacuum to afford the target product 4-ethoxy-2-fluorobenzaldehyde (1.0 g, 83% yield) as a red solid.1H-NMR (CD3OD, 400 MHz) data were as follows: δ 10.09 (s, 1H), 7.76-7.67 (m, 1H), 6.83-6.74 (m, 2H), 4.11 (t, J = 6.8 Hz, 2H), 1.40 (t, J = 7.0 Hz, 3H).

348-27-6
205 suppliers
$15.00/1g

75-03-6
369 suppliers
$11.00/5g

532965-74-5
34 suppliers
$185.00/1g
Yield:532965-74-5 83%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 25 - 60; for 10 h;Inert atmosphere;
Steps:
136B
To a mixture of 2-fluoro-4-hydroxy-benzaldehyde (1.0 g, 7.1 mmol) and potassium carbonate (2.0 g, 14 mmol) in N, N-dimethylformamide ( 10 mL) at 25 °C under nitrogen was added iodoethane (1.1 g, 7.1 mmol). The mixture was stirred at 60 °C for 10 hours, concentrated in vacuo, diluted with ethyl acetate (200 mL), washed with saturated sodium bicarbonate solution (3 x 50 mL) and brine (3 x 50 mL), dried with anhydrous sodium sulfate and concentrated in vacuo to give compound B-238 (1.0 g, 83% yield) as a red solid. -NMR (CD3OD, 400 MHz): 10.09 (s, 7.76-7.67 (m, IH), 6.83-6.74 (m, 2H), 4.11 (t, J=6.8 Hz, 2H), 1.40 (t, J=7.0 Hz, 3H).
References:
WO2016/100184,2016,A1 Location in patent:Paragraph 00537-00538