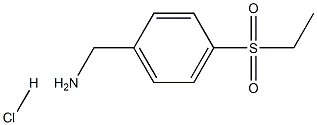
(4-(ethylsulfonyl)phenyl)methanamine hydrochloride synthesis
- Product Name:(4-(ethylsulfonyl)phenyl)methanamine hydrochloride
- CAS Number:98959-89-8
- Molecular formula:C9H14ClNO2S
- Molecular Weight:235.731

409112-19-2

98959-89-8
To a stirring solution of 4-(ethylsulfonyl)benzonitrile (1.0 g, 5.12 mmol) in methanol (30 mL) was sequentially added nickel ruanne (500 mg) and ammonia (2.0 mL) at room temperature. The reaction mixture was transferred to a Parr reactor and hydrogenated at 60 psi hydrogen pressure for 1 hour. Upon completion of the reaction, the mixture was filtered through a bed of diatomaceous earth and the filtrate was concentrated under reduced pressure. The resulting crude product was purified by silica gel column chromatography. Subsequently, the purified free base was reacted with ethyl acetate solution of hydrochloric acid with stirring to give 513 mg of the target product [4-(ethylsulfonyl)phenyl]methylamine hydrochloride. The product characterization data were as follows: nMR (300 MHz, DMSO-d6) δ 1.09 (t, J = 7.5 Hz, 3H), 3.32 (q, J = 7.5 Hz, 2H), 4.15 (s, 2H), 7.76 (d, J = 7.2 Hz, 2H), 7.93 (d, J = 8.4 Hz, 2H), 8.50 (br s, 2H). ESI-MS (m/z) 200 ([M + H]+).

409112-19-2
12 suppliers
inquiry

98959-89-8
24 suppliers
inquiry
Yield:98959-89-8 531 mg
Reaction Conditions:
with ammonia;hydrogen in methanol;water; under 3102.97 Torr; for 1 h;
Steps:
5 Step 5: (4-(Ethylsulfonyl)phenyl)methanamine hydrochloride
To a stirred solution of 4-(ethylsulfonyl)benzonitrile (Step 4 intermediate) (1.0 g, 5.12 mmol) in methanol (30 mL) was added Raney nickel (500 mg) and aqueous ammonia (2.0 mL) at RT. The mixture was subjected to hydrogenation in a Parr apparatus under 60 psi hydrogen pressure for 1 h. The reaction mixture was filtered through celite bed and the filtrate was concentrated under reduced pressure. The residue obtained was purified by silica gel column chromatography. The free base was stirred with hydrochloric acid in ethyl acetate to yield 513 mg of the desired product as HC1 salt. NMR (300 MHz, DMSO-Je) δ 1.09 (t, = 7.5 Hz, 3H), 3.32 (q, = 7.5 Hz, 2H), 4.15 (s, 2H), 7.76 (d, = 7.2 Hz, 2H), 7.93 (d, = 8.4 Hz, 2H), 8.50 (br s, 2H); ESI-MS (m/z) 200 (M+H)+.
References:
WO2018/116285,2018,A1 Location in patent:Page/Page column 20; 22-23

90561-19-6
9 suppliers
inquiry

98959-89-8
24 suppliers
inquiry
![[4-(Ethanesulphonyl)phenyl]methanamine](/CAS/GIF/583837-94-9.gif)
583837-94-9
39 suppliers
$211.00/250mg

98959-89-8
24 suppliers
inquiry
824-79-3
420 suppliers
$6.00/25g

98959-89-8
24 suppliers
inquiry
7569-34-8
26 suppliers
$479.00/10g

98959-89-8
24 suppliers
inquiry