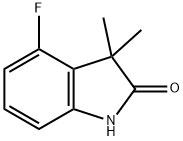
4-fluoro-1,3-dihydro-3,3-diMethyl-2H-Indol-2-one synthesis
- Product Name:4-fluoro-1,3-dihydro-3,3-diMethyl-2H-Indol-2-one
- CAS Number:866211-50-9
- Molecular formula:C10H10FNO
- Molecular Weight:179.19
138343-94-9
116 suppliers
$35.00/250mg

74-88-4
341 suppliers
$15.00/10g

866211-50-9
12 suppliers
inquiry
Yield:866211-50-9 25%
Reaction Conditions:
Stage #1: 4-fluoro-1,3-dihydro-2H-indol-2-onewith n-butyllithium;lithium chloride in tetrahydrofuran;hexane at -78; for 0.25 h;
Stage #2: methyl iodide in tetrahydrofuran;hexane at 20; for 16 h;Further stages.;
References:
Fensome, Andrew;Adams, William R.;Adams, Andrea L.;Berrodin, Tom J.;Cohen, Jeff;Huselton, Christine;Illenberger, Arthur;Kern, Jeffrey C.;Hudak, Valerie A.;Marella, Michael A.;Melenski, Edward G.;McComas, Casey C.;Mugford, Cheryl A.;Slayden, Ov D.;Yudt, Matthew;Zhang, Zhiming;Zhang, Puwen;Zhu, Yuan;Winneker, Richard C.;Wrobel, Jay E. [Journal of Medicinal Chemistry,2008,vol. 51,# 6,p. 1861 - 1873]