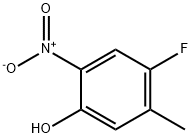
4-Fluoro-5-methyl-2-nitrophenol synthesis
- Product Name:4-Fluoro-5-methyl-2-nitrophenol
- CAS Number:182880-62-2
- Molecular formula:C7H6FNO3
- Molecular Weight:171.13
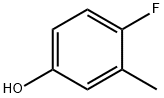
452-70-0
233 suppliers
$10.00/2g

182880-62-2
33 suppliers
inquiry
Yield:182880-62-2 33%
Reaction Conditions:
with tetrabutylammomium bromide;nitric acid in water;1,2-dichloro-ethane at 20; for 3 h;
Steps:
4-Fl uoro-5-methyl-2-nitrophenol
To a solution of 4-fluoro-3-methylphenol (bOg, 79.3mmol), in DOE: H20 (1:2, 150 mL) were added TBAB (2.6 g, 7.93 mmol) and HNO3 (6.6 mL, 15.9mmol) at rt. The reaction mixture was stirred at rt for 3h. TLC showed the reaction to be complete. The reaction mixture was poured in to ice-water (lOOmL) and extracted with DCM (3xlOOmL). The organic layer was washed with brine (lOOmL), dried (Na2504), filtered and concentrated under reduced pressure. The crude residue was purified by column chromatography using silica gel (100-200 mesh), eluting with 5% EtOAC in hexane to give 4-fluoro-5-methyl-2-nitrophenol as a yellow solid. Yield: 4.5 g (33%); 1H NMR (400 MHz, DMSO-d6): 10.81 (bs, 1H), 7.73-7.77 (m, 1H), 7.01- 7.05 (m, 1H), 2.32(s, 3H); MS (ESI-) for CHNOS m/z 170.05 [M-H]. The formation of exact regioisomer was further confirmed by fluorine decoupled NMR.
References:
WO2018/37223,2018,A1 Location in patent:Page/Page column 203