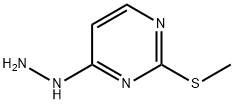
4-Hydrazino-2-(methylsulfanyl)pyrimidine synthesis
- Product Name:4-Hydrazino-2-(methylsulfanyl)pyrimidine
- CAS Number:104408-29-9
- Molecular formula:C5H8N4S
- Molecular Weight:156.21
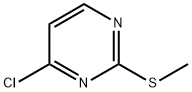
49844-90-8

104408-29-9
The general procedure for the synthesis of 4-hydrazino-2-methylthiopyrimidine from 2-methylthio-4-chloropyrimidine is as follows: Step 1: Preparation of N-(2-methylthiopyrimidin-4-yl)hydrazine In a 250 mL round bottom flask, 4-chloro-2-methylthiopyrimidine (5 g, 31.1 mmol), hydrazine hydrate (4.5 g, 140 mmol) and potassium carbonate (6.45 g, 46.7 mmol) were dissolved in ethanol (50 mL). The reaction mixture was heated to reflux for 3 h. After the reaction was completed, it was filtered while hot. The filter cake was washed with ethanol (30 mL). The filtrate and washings were combined and concentrated under reduced pressure to remove the solvent to give the crude product. Purification of the crude product by ISCO fast column chromatography (eluent: 30% to 100% hexane solution of ethyl acetate) afforded N-(2-methylthiopyrimidin-4-yl)hydrazine as a white solid (2.8 g, 57.6% yield).

49844-90-8
251 suppliers
$5.00/1g

104408-29-9
35 suppliers
inquiry
Yield:104408-29-9 57.6%
Reaction Conditions:
with potassium carbonate;hydrazine in ethanol; for 3 h;Reflux;
Steps:
1
Trans-4-{4-[4-(4-Methanesulfonyl-piperidin-1-yl)-indazol-1-yl]-pyrimidin-2-ylamino}-cyclohexanol; Step 1: Preparation of N-(2-methylsulfanyl-pyrimidin-4-yl)-hydrazine; In a 250 mL round bottom flask, 4-chloro-2-methylsulfanyl-pyrimidine (5 g, 31.1 mmol), hydrazine (4.5 g, 140 mmol) and potassium carbonate (6.45 g, 46.7 mmol) were combined with ethanol (50 mL). The mixture was refluxed for 3 hrs and filtered. The filtered solid was rinsed with ethanol (30 mL). The combined ethanol solution was evaporated to give a crude oily mixture which was purified by ISCO flash column chromatography (30% to 100% ethyl acetate in hexanes) to give N-(2-methylsulfanyl-pyrimidin-4-yl)-hydrazine as a white solid (2.8 g, 57.6% yield).
References:
US2011/34470,2011,A1 Location in patent:Page/Page column 40