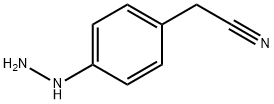
(4-hydrazinophenyl)acetonitrile synthesis
- Product Name:(4-hydrazinophenyl)acetonitrile
- CAS Number:57411-91-3
- Molecular formula:C8H9N3
- Molecular Weight:147.18
3544-25-0
288 suppliers
$10.00/5g

57411-91-3
9 suppliers
inquiry
Yield:57411-91-3 100%
Reaction Conditions:
with sodium nitrite in hydrogenchloride;
Steps:
1.1 1.
1. 4-Hydrazinobenzylcyanide. Hydrochloride A solution of NaNO2 (80 g, 1.16 mol) was added dropwise to a cooled (-10° C.), stirred, suspension of 4-aminobenzyl cyanide (153.5 g, 1.16 mol) in concentrated HCl (1500 ml), at such a rate that the temperature did not rise above -10° C. The mixture was stirred at -10° C. for 0.25 h before being filtered rapidly under vacuum into an addition funnel. The solution was added portionwise over a 0.25 h period to a rapidly stirred mixture of SnCl2.2H2 O (1.05 kg, 4.64 mol) in concentrated HCl (800 ml) keeping the temperature below -5° C. The mixture was allowed to warm to room temperature and stir for 0.25 h before filtering the sandy coloured precipitate under vacuum and washing with ether (5*500 ml). The resultant solid was dried over P2 O5 in a vacuum oven (80° C.) for 16 h to give the title compound (213 g, 100%), m.p. 181°-183° C.; 1 H NMR (360 MHz, D2 O) δ 3.90 (2H, s, CH2); 7.06 (2H, d, J=8.7 Hz, Ar-H); 7.40 (2H, d, J=8.7 Hz, Ar-H).
References:
US5298520,1994,A