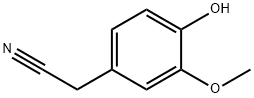
4-HYDROXY-3-METHOXYPHENYLACETONITRILE synthesis
- Product Name:4-HYDROXY-3-METHOXYPHENYLACETONITRILE
- CAS Number:4468-59-1
- Molecular formula:C9H9NO2
- Molecular Weight:163.17
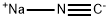
773837-37-9
0 suppliers
inquiry
498-00-0
357 suppliers
$21.00/25 g

4468-59-1
76 suppliers
$23.00/250 mg
Yield: 68%
Reaction Conditions:
in N,N-dimethyl-formamide at 120; for 24 h;Inert atmosphere;
Steps:
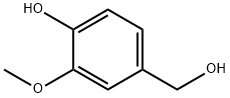
2'-(4-Hydroxy-3-methoxyphenyl)acetonitrile 17
To a solution of alcohol 16 (18 g, 0.12 mol) in DMF (300 mL), under an atmosphere of nitrogen was added NaCN (6.9 g, 0.14 mol), and the mixture was heated at 120°C and stirred for 24 h. The solution was cooled to room temperature and water (100 mL) was added cautiously. The reaction mixturewas basified (solid NaOH) to pH 10 and the DMF was removed by distillation. Water (250 mL) and acetic acid (20 mL) were added until a neutral pH (~7) was achieved. The aqueous mixture was extracted with chloroform (5x100 mL) and the combined organic extracts were washed with water (5x50 mL), dried (MgSO4), and the solvent was removed under reduced pressure to give the title compound (13 g, 68%, quantitative brsm.) as a brown oil which was used without purification in the subsequent reaction. RF (2:1 hexanes, ethyl acetate) 0.33; dH (400 MHz; CDCl3; Me4Si) 3.68 (2H, s, CH2Ar), 3.90 (3H, s, OCH3), 5.73 (1H, br s, OH), 6.81-6.90 (3H, m, 2-, 5- and 6-H); Spectroscopic data were in accordance with literature values.
References:
Dittrich, Nora;Pilkington, Lisa I.;Leung, Euphemia;Barker, David [Tetrahedron,2017,vol. 73,# 14,p. 1881 - 1894]
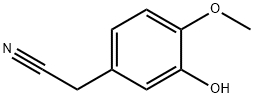
4468-58-0
14 suppliers
inquiry

4468-59-1
76 suppliers
$23.00/250 mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
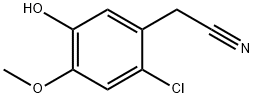
81038-46-2
0 suppliers
inquiry

498-00-0
357 suppliers
$21.00/25 g

4468-59-1
76 suppliers
$23.00/250 mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
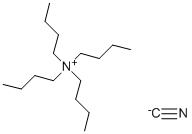
10442-39-4
62 suppliers
$45.00/250mg

4468-59-1
76 suppliers
$23.00/250 mg
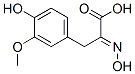
5447-36-9
1 suppliers
inquiry

4468-59-1
76 suppliers
$23.00/250 mg