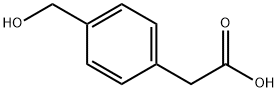
4-(HYDROXYMETHYL)PHENYLACETIC ACID synthesis
- Product Name:4-(HYDROXYMETHYL)PHENYLACETIC ACID
- CAS Number:73401-74-8
- Molecular formula:C9H10O3
- Molecular Weight:166.17
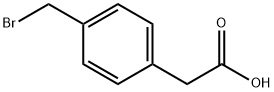
13737-36-5

73401-74-8
The general procedure for the synthesis of 4-(hydroxymethyl)benzeneacetic acid from 4-bromomethylphenylacetic acid was as follows: 4-bromomethylphenylacetic acid (2.3 kg, 10.0 mol, 1 eq.) was added to an aqueous (90 L) solution of sodium hydroxide (1.61 kg, 40.2 mol, 4 eq.) and the resulting mixture was stirred at room temperature overnight. After confirming the complete consumption of 4-bromomethylphenylacetic acid by TLC analysis, the reaction mixture was carefully acidified with concentrated sulfuric acid (1.0 L) to a pH of about 2. Subsequently, solid sodium chloride (25 kg) was added to the mixture, followed by extraction with ethyl acetate (33 L × 3). The combined organic phases were washed with brine, dried over anhydrous sodium sulfate, and concentrated until a significant amount of solid precipitated. The resulting suspension was kept at 4-6 °C overnight to promote further crystallization. The solid product was collected by filtration and the filter cake was washed with petroleum ether (2 L × 2) to afford 4-(hydroxymethyl)benzeneacetic acid (1.2 kg, yield: 71.9%). HPLC purity was 97.8% (220 nm); 1H NMR (300 MHz, DMSO-d6) δ 12.27 (s, 1H), 7.26-7.12 (m, 4H), 5.14 ( s, 1H), 4.47 (s, 2H), 3.53 (s, 2H).

13737-36-5
257 suppliers
$10.40/1G

73401-74-8
200 suppliers
$14.56/1G
Yield:73401-74-8 71.9%
Reaction Conditions:
with sodium hydroxide in water at 18 - 25;Large scale;
Steps:
1 2-(4-(Hydroxymethyl)phenyl)acetic acid (C)
2-(4-(Hydroxymethyl)phenyl)acetic acid (C)
To a solution of NaOH (1.61 kg, 40.2 mol, 4 eq) in water (90 L) was added B (2.3 kg, 10.0 mol, 1 eq) and the resulting mixture was stirred at RT overnight. TLC analysis indicated consumption of B.
The reaction mixture was then carefully acidified with concentrated H2SO4 (1.0 L) to pH?2.
Then, solid NaCl (25 kg) was added to the mixture followed by extraction with EtOAc (33 L*3).
The combined organic phase was washed with brine, dried over Na2SO4, and concentrated until a significant amount of solid precipitated.
The resulting suspension was kept at ?4-6° C. overnight to allow for further crystallization.
The solid product was then collected by filtration.
The filter cake was washed with petroleum ether (2 L*2) to yield the title compound (1.2 kg, yield: 71.9%). HPLC purity: 97.8% (220 nm); 1H NMR (300 MHz, DMSO-d6) δ 12.27 (s, 1H), 7.26-7.12 (m, 4H), 5.14 (s, 1H), 4.47 (s, 2H), 3.53 (s, 2H).
References:
US9643927,2017,B1 Location in patent:Page/Page column 33;34
622-47-9
416 suppliers
$17.00/25g

73401-74-8
200 suppliers
$14.56/1G