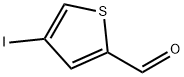
4-Iodo-2-thiophenecarbaldehyde synthesis
- Product Name:4-Iodo-2-thiophenecarbaldehyde
- CAS Number:18812-38-9
- Molecular formula:C5H3IOS
- Molecular Weight:238.05
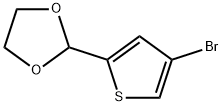
58267-85-9

18812-38-9
General procedure for the synthesis of 4-iodothiophene-2-carbaldehyde using 2-(4-bromothiophen-2-yl)-1,3-dioxolane as starting material: 2-(4-bromothiophen-2-yl)-1,3-dioxolane (8.02 g, 34 mmol) was dissolved in anhydrous ethyl ether (150 mL), and cooled to -78°C. A hexane solution of 2.5 M n-butyllithium (16.3 mL, 41 mmol) was slowly added dropwise with stirring, and stirring was continued for 20 min after completion of the drop. Subsequently, an anhydrous ether solution (50 mL) of iodine (8.64 g, 34 mmol) was added slowly dropwise, and the solution gradually turned red during the dropwise addition. After the dropwise addition, the reaction mixture was slowly warmed up to room temperature and stirring was continued for 20 min. The reaction was quenched by the addition of 1N hydrochloric acid solution (100 mL) and stirred overnight. After completion of the reaction, the reaction mixture was diluted with ethyl acetate and partitioned with deionized water. The organic phase was dried with anhydrous magnesium sulfate and concentrated under reduced pressure to remove the solvent to give a yellow oily product (7.0 g, 87% yield). The structure of the product was confirmed by 1H NMR (300 MHz, DMSO-D6): δ 8.10 (d, J = 1.32 Hz, 1H), 8.33 (t, J = 1.22 Hz, 1H), 9.90 (d, J = 1.32 Hz, 1H).

58267-85-9
39 suppliers
$15.00/1g

18812-38-9
32 suppliers
$74.00/100mg
Yield:18812-38-9 87%
Reaction Conditions:
Stage #1: 2-(4-bromo-thiophen-2-yl)-[1,3]dioxolanewith n-butyllithium in diethyl ether;hexane at -78; for 0.333333 h;
Stage #2: with iodine in diethyl ether;hexane at 20; for 0.333333 h;
Stage #3: with hydrogenchloride in diethyl ether;hexane;water;
Steps:
23.2
A solution of 2-(4-bromothien-2-yl)- 1,3-dioxolane (8.02 g, 34 mmol) in ether (150 mL, anhydrous) was cooled to -78 followed by the dropwise addition of 2.5 M n-BuLi in hexanes (16.3 mL, 41 mmol). The reaction was stirred for 20 minutes. A solution of iodine (8.64 g, 34 mmol) in ether (50 mL, anhydrous) was added dropwise resulting in a red solution upon addition of the last drop. The reaction was warmed to room temperature and stirred for 20 minutes. IN HCl (100 mL) was added and the reaction was stirred overnight. The reaction mixture was diluted with EtOAc and partitioned with water. Drying (MgS04) and removal of solvent gave a yellow oil (7.0 g, 87%). H NMR (300 MHz, DMSO-D6) 5 ppm 8.10 (d, J=1.32 Hz, 1 H) 8.33 (t, J=1.22 Hz, 1 H) 9.90 (d, J=1.32 Hz, 1 H).
References:
WO2005/111001,2005,A1 Location in patent:Page/Page column 47