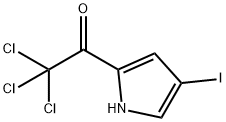
4-IODO-2-(TRICHLOROACETYL)PYRROLE synthesis
- Product Name:4-IODO-2-(TRICHLOROACETYL)PYRROLE
- CAS Number:72652-33-6
- Molecular formula:C6H3Cl3INO
- Molecular Weight:338.36
35302-72-8
174 suppliers
$10.00/5g

72652-33-6
36 suppliers
$137.84/1gm:
Yield:72652-33-6 94%
Reaction Conditions:
with iodine;(2,2,2-trifluoroacetyl)oxysilver in dichloromethane at 0 - 18;
Steps:
Iodine (1.2 g, 4.7 mmol) was added portion wise to a solution of 2,2,2-trichloro-1-(1 H-pyrrol-2-yl) ethanone (1 g, 4.7 mmol) and silver trifluoroacetate (1.1 g, 5 mmol) in dry dichloromethane (24 mL), cooled to 0 0C. The reaction mixture was allowed to warm up slowly to 18 0C (water bath) and stirred at the same temperature for 5 h. The solid was filtered, the organic phase washed with Na2S2Os (5% aq. solution) until decoloration occurs and finally washed with H2O (1 x 20 mL). The organic phase was dried over Na2SU4 and filtered through a plough of SiO2 (hexane- EtOAc 4:1), to obtain compound 2,2,2-trichloro-1-(4-iodo-1H-pyrrol-2-yl)ethanone (IX) as an off-white solid (1.49 g, 94% yield).LCMS (HPLC Method 2): m/z 336 [M-H]- (at) r.t. 6.3 min. 1H NMR (400 MHz, DMSO-d6) δ = 12.76 (br. s., 1 H), 7.52 (dd, J = 1.3, 3.3 Hz, 1 H), 7.39 (dd, J = 1.3, 2.6 Hz, 1 H).
References:
WO2010/31816,2010,A1 Location in patent:Page/Page column 48