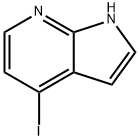
4-IODO-7-AZAINDOLE synthesis
- Product Name:4-IODO-7-AZAINDOLE
- CAS Number:319474-34-5
- Molecular formula:C7H5IN2
- Molecular Weight:244.03
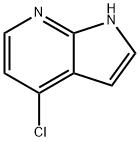
55052-28-3

319474-34-5
Part 2: Synthesis of 4-iodo-1H-pyrrolo[2,3-b]pyridine: To a mixture of 4-chloro-1H-pyrrolo[2,3-b]pyridine (12.9 g, 84.3 mmol) and NaI (40 g, 168 mmol) in acetonitrile (150 mL) was slowly added acetyl chloride (12.6 mL, 176 mmol). The reaction mixture was stirred at 80 °C for 4 days, followed by removal of excess acetonitrile by distillation under reduced pressure. To the residue was added 300 mL of 10% K2CO3 (aqueous solution) and extracted with CH2Cl2 (3 x 100 mL). The combined organic phases were washed sequentially with 10% sodium bisulfite (aqueous) and saturated brine, dried over anhydrous sodium sulfate and concentrated under reduced pressure to give the crude product (22.2 g). The crude product was dissolved in THF (150 mL) and 1 M NaOH (100 mL) was added. The mixture was stirred at room temperature for 2 h. The solvent was subsequently evaporated under reduced pressure, diluted with water and extracted with CH2Cl2. The organic phase was washed with saturated saline, dried over anhydrous sodium sulfate and concentrated under reduced pressure. The resulting brown solid was purified by silica gel column chromatography and recrystallized with acetonitrile to give pure 4-iodo-1H-pyrrolo[2,3-b]pyridine (9.75 g, 48% yield). Mass spectrum (ES+): 245 [MH+].

75-36-5
589 suppliers
$17.92/100G

55052-28-3
477 suppliers
$6.00/1g

319474-34-5
113 suppliers
$14.00/250mg
![Ethanone, 1-(4-iodo-1H-pyrrolo[2,3-b]pyridin-1-yl)-](/CAS/GIF/443729-67-7.gif)
443729-67-7
16 suppliers
$152.00/1mg
Yield: 80%
Reaction Conditions:
Stage #1:acetyl chloride;4-chloro-7-azaindole with sodium iodide in acetonitrile at 20 - 85; for 12 h;
Stage #2: with sodium carbonate;sodium hydrogensulfite in water at 20; for 0.25 h;
Steps:
3.1
A solution of meta-chlorobenzoic acid (14 g, 54 mmol) in ethyl acetate (30 mL) was dropwise added to a solution of 1H-pyrrolo[2,3-b]pyridine (5.3 g, 45 mmol) in ethyl acetate (45 mL) over 1 hr with stirring at 0°C. After completion of the dropping, the mixture was stirred at room temperature for 3 hr, followed by leaving to stand at 0°C. The resulting crystals were collected by filtration, washed with ethyl acetate, and then dried under reduced pressure. The crystals were dissolved in water (30 mL), and then 30% K2CO3 was added until the pH of the solution reached 10. The solution was left to stand at room temperature for 1 hr and then at 0°C for 1 hr. The resulting precipitate was collected by filtration and was washed with ether to yield 3.5 g (58%) of N-oxide. The N-oxide (3.0 g, 22 mmol) was dissolved in DMF (16 mL). The resulting solution was heated at 50°C, and a solution of methanesulfonyl chloride (4.7 mL, 60 mmol) in DMF (6.4 mL) was dropwise added to the solution at 70°C. This reaction solution was stirred at 75°C for 2 hr. The reaction solution was added to ice and was neutralized with 10 N NaOH at 0°C, followed by stirring at room temperature for 1 hr. The resulting precipitate was collected by filtration, washed with water, and dried at 60°C under reduced pressure to yield 2.7 g (80%) of the target 4-chloro-1H-pyrrolo[2,3-b]pyridine. 4-Chloro-1H-pyrrolo[2,3-b]pyridine (2.7 g, 18 mmol) and NaI (13 g, 88 mmol) were dissolved in acetonitrile (28 mL), and CH3COCl (3.5 mL, 50 mmol) was added thereto with stirring at room temperature. The reaction solution was heated at 85°C for 12 hr and then cooled to room temperature, and 10% Na2CO3 (28 mL) and 10% NaHSO3 (28 mL) were added thereto, followed by stirring at room temperature for 15 min. Ethyl acetate was added thereto for separation, and the organic layer was washed with saturated brine. The organic layer was dried over anhydrous sodium sulfate, concentrated, and purified with a silica gel column to yield 4-iodo-1-N-acetyl-pyrrolo[2,3-b]pyridine (2.0 g) and 4-iodo-1H-pyrrolo[2,3-b]pyridine (2.3 g). 4-Iodo-1-N-acetyl-pyrrolo[2,3-b]pyridine (2.0 g, 7.0 mmol) was dissolved in ethanol (70 mL) and refluxed in methanol containing 28% sodium methoxide (1.4 mL, 7.0 mmol) for 1 hr. The reaction solution was concentrated and separated between ethyl acetate and an aqueous saturated ammonium chloride solution. The organic layer was washed with an aqueous saturated ammonium chloride solution, dried over anhydrous sodium sulfate, concentrated, and then combined with 4-iodo-1H-pyrrolo[2,3-b]pyridine (2.3 g) obtained above. The mixture was recrystallized from ethanol to yield 4-iodo-1H-pyrrolo[2,3-b]pyridine (4.0 g, 92%). 4-Iodo-1H-pyrrolo[2,3-b]pyridine: 1H NMR (500 MHz, DMSO-d6) δ 12.01 (s, 1H), 7.89 (d, 1H, J = 5.0 Hz), 7.59 (t, 1H, J = 3.1 Hz), 7.51 (d, 1H, J = 5.0 Hz), 6.27 (d, 1H, J = 3.4 Hz).
References:
Riken;TagCyx Biotechnologies EP2487180, 2012, A1 Location in patent:Page/Page column 20

55052-28-3
477 suppliers
$6.00/1g

319474-34-5
113 suppliers
$14.00/250mg
![Ethanone, 1-(4-iodo-1H-pyrrolo[2,3-b]pyridin-1-yl)-](/CAS/GIF/443729-67-7.gif)
443729-67-7
16 suppliers
$152.00/1mg

319474-34-5
113 suppliers
$14.00/250mg
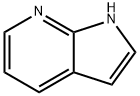
271-63-6
573 suppliers
$9.00/5g

319474-34-5
113 suppliers
$14.00/250mg