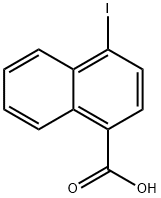
4-Iodonaphthalene-1-carboxylic acid synthesis
- Product Name:4-Iodonaphthalene-1-carboxylic acid
- CAS Number:91059-41-5
- Molecular formula:C11H7IO2
- Molecular Weight:298.08
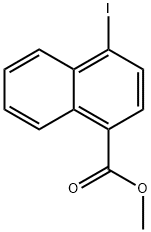
402476-02-2
1 suppliers
inquiry

91059-41-5
5 suppliers
inquiry
Yield:91059-41-5 86%
Reaction Conditions:
Stage #1: 1-iodo-4-methoxycarbonylnaphthalenewith potassium hydroxide in water; for 12 h;Inert atmosphere;Reflux;
Stage #2: with hydrogenchloride in water;
Steps:
5.1.6. 4-Chloro-1-naphthoic acid (22, X = Cl)
General procedure: To a solution of 2.44 g (11.9 mmol) of 1-acetyl-4-chloronaphthalene in 5 mL of freshly distilled pyridine under N2 was added 3.33 g (13.1 mmol) of I2 dissolved in 15 mL of freshly distilled pyridine. The mixture was heated at reflux for 40 min, cooled to ambient temperature and diluted with ether until a brown precipitate formed. The precipitate was filtered off, suspended in 12 mL of 6 M aqueous NaOH and heated at reflux for 2 h. After cooling the solution was acidified with 10% HCl, the crude 4-chloro-1-naphthoic acid was extracted into ether and the ethereal extract was washed with brine. After drying (MgSO4) the solution was concentrated in vacuo. The product was dissolved in 25 mL of MeOH to which 5 mL of concentrated H2SO4 was cautiously added. The solution was heated at reflux for 3 h. After cooling to ambient temperature the crude ester was extracted into ether, the ethereal solution was washed with brine and dried (MgSO4). The solution was concentrated in vacuo to give 1.66 g (63%) of methyl 4-chloro-1-naphthoate as a brown oil, which was used without further purification: 1H NMR (300 MHz, CDCl3) δ 3.90 (s, 3H), 7.38 (d, J = 7.8, 1H), 7.45-7.56 (m, 2H), 7.87 (d, J = 8.1, 1H), 8.18 (d, J = 8.4, 1H), 8.93 (d, J = 8.1, 1H); 13C NMR (75.5 MHz, CDCl3) δ 52.1, 124.6, 124.9, 125.8. 126.2, 127.1, 128.3, 129.9, 130.7, 132.3, 137.2, 166.9; GC/MS (EI) m/z (rel intensity) 220 (61), 189 (100), 161 (48), 126 (42).A mixture of 0.25 g (1.1 mmol) of methyl 4-chloro-1-naphthoate and 2.10 g (37.4 mmol) of KOH in 20 mL of H2O was heated at reflux for 12 h under N2. The reaction mixture was cooled to ambient temperature, acidified with concentrated HCl and the product was extracted into ether and dried (MgSO4). The organic phase was concentrated in vacuo to give 0.27 g (88%) of 4-chloro-1-naphthoic acid as an off-white solid
References:
Wiley, Jenny L.;Smith, Valerie J.;Chen, Jianhong;Martin, Billy R.;Huffman, John W. [Bioorganic and Medicinal Chemistry,2012,vol. 20,# 6,p. 2067 - 2081] Location in patent:experimental part
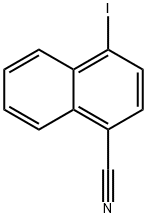
140456-96-8
6 suppliers
inquiry

91059-41-5
5 suppliers
inquiry
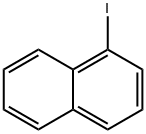
90-14-2
298 suppliers
$19.00/5g

91059-41-5
5 suppliers
inquiry
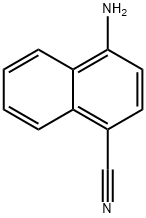
58728-64-6
74 suppliers
$78.00/100mg

91059-41-5
5 suppliers
inquiry

91493-16-2
1 suppliers
inquiry

91059-41-5
5 suppliers
inquiry