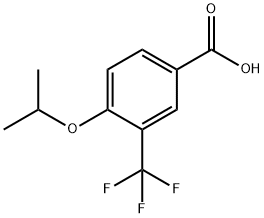
4-isopropoxy-3-(trifluoroMethyl)benzoic acid synthesis
- Product Name:4-isopropoxy-3-(trifluoroMethyl)benzoic acid
- CAS Number:213598-16-4
- Molecular formula:C11H11F3O3
- Molecular Weight:248.2
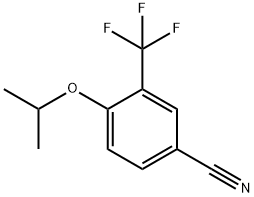
1035217-13-0

213598-16-4
To a solution of 4-isopropoxy-3-(trifluoromethyl)benzonitrile (183 g, 798 mmol) in ethanol (1 L) was added 5 N sodium hydroxide solution (559 mL, 2.80 mol). The reaction mixture was heated to 80 °C and kept for 18 hours. Upon completion of the reaction, the volatiles were removed by vacuum and 3 N hydrochloric acid was added to the residue until the mixture became acidic. At this point a precipitate was formed and the precipitate was collected by vacuum filtration. The solid was washed with water and hexane. The solid was dissolved in ethyl acetate, dried over magnesium sulfate, filtered and concentrated to give 4-isopropoxy-3-(trifluoromethyl)benzoic acid (191 g, 770 mmol, 96% yield) as a white solid. The calculated value for the exact mass was 248.1, measured value: LCMS m/z = 249.3 [M + H]+; 1H NMR (400 MHz, CDCl3) δ ppm 1.41 (d, J = 6.0 Hz, 6H), 4.76 (sep, J = 6.0 Hz, 1H), 7.05 (d, J = 8.8 Hz, 1H), 8.22 (dd, J1 = 8.8 Hz, J2 = 2.2 Hz, 1H), 8.33 (d, J = 2.0 Hz, 1H).

1035217-13-0
5 suppliers
inquiry

213598-16-4
36 suppliers
$35.00/100mg
Yield:213598-16-4 96%
Reaction Conditions:
Stage #1: 4-isopropoxy-3-(trifluoromethyl)benzonitrilewith water;sodium hydroxide in ethanol at 80; for 18 h;
Stage #2: with hydrogenchloride in water;
Steps:
1.1.B
To a solution of 4-isopropoxy-3-(trifluoromethyl)benzonitrile (183 g, 798 mmol) in EtOH (1 L) was added 5 N NaOH (559 mL, 2.80 mol). The reaction mixture was heated to 80 °C for 18 h. Volatiles were removed in vacuo and 3 N HCl was added until the mixture was acidic. A precipitate formed that was collected by vacuum filtration. The solid was washed with water and hexanes. The solid was dissolved in EtOAc and dried over MgS04 to give 4-isopropoxy-3- (trifluoromethyl)benzoic acid (191 g, 770 mmol, 96% yield) as a white solid. Exact mass calculated for 248.1, found: LCMS m/z = 249.3, [M+H+]; lU NMR (400 MHz, CDC13) δ ppm 1.41 (d, = 6.0 Hz, 6 H), 4.76 (sep, = 6.0 Hz, 1 H), 7.05 (d, = 8.8 Hz, 1 H), 8.22 (dd, Jx = 8.8, J2 = 2.2 Hz, 1 H), 8.33 (d, = 2.0 Hz, 1 H).
References:
WO2011/109471,2011,A1 Location in patent:Page/Page column 111
1034188-33-4
1 suppliers
inquiry

213598-16-4
36 suppliers
$35.00/100mg
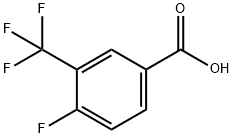
67515-55-3
211 suppliers
$10.00/1g
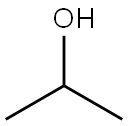
67-63-0
1640 suppliers
$17.00/25ML

213598-16-4
36 suppliers
$35.00/100mg
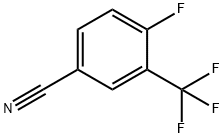
67515-59-7
219 suppliers
$9.00/1g

213598-16-4
36 suppliers
$35.00/100mg
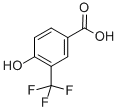
220239-68-9
101 suppliers
$9.00/250mg

213598-16-4
36 suppliers
$35.00/100mg