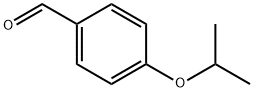
4-ISOPROPOXYBENZALDEHYDE synthesis
- Product Name:4-ISOPROPOXYBENZALDEHYDE
- CAS Number:18962-05-5
- Molecular formula:C10H12O2
- Molecular Weight:164.2
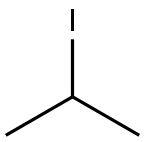
75-30-9
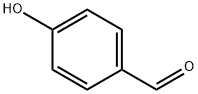
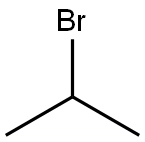
123-08-0

18962-05-5
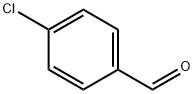
General procedure for the synthesis of 4-isopropoxybenzaldehyde from 2-iodopropane and p-hydroxybenzaldehyde: Referring to Example 3, to a 100 mL DMF solution containing 2 g (16.38 mmol) of p-hydroxybenzaldehyde was added sequentially 2.72 g (19.69 mmol) of K2CO3, 2.74 g (16.52 mmol) of KI, and 3.94 mL ( 39.38 mmol) 2-iodopropane and the reaction was carried out under argon protection. The reaction mixture was stirred at 80 °C overnight and subsequently concentrated. The concentrated residue was partitioned between CHCl3 and H2O to separate the organic and aqueous phases. The aqueous phase was further extracted with CHCl3 and after combining all the organic phases was dried and concentrated with MgSO4. The crude product was purified by silica gel column chromatography using a gradient polarity EtOAc/hexane mixed solvent as eluent, resulting in 2.08 g of oily 4-isopropoxybenzaldehyde in 77% yield. The structure of the product was confirmed by 1H-NMR (300 MHz, CDCl3, δTMS): 1.39 (d, J=6Hz, 6H), 4.67 (m, 1H), 6.96 (d, J=8.7Hz, 2H), 7.81 (d, J=8.7Hz, 2H), 9.87 (s, 1H).
Yield:18962-05-5 77%
Reaction Conditions:
with potassium carbonate in hexane;ethyl acetate;N,N-dimethyl-formamide;
Steps:
R.3 4-Isopropoxybenzaldehyde
REFERENCE EXAMPLE 3 4-Isopropoxybenzaldehyde To a solution of 2 g (16.38 mmol) of 4-hydroxybenzaldehyde in 100 mL of DMF, 2.72 g (19.69 mmol) of K2CO3, 2.74 g (16.52 mmol) of KI and 3.94 mL (39.38 mmol) of 2-iodopropane was added under argon. The mixture was stirred at 80° C. overnight, concentrated and the residue obtained was partitioned between CHCl3 and H2O. The phases were separated, the aqueous phase was extracted with CHCl3 and the combined organic phases were dried over MgSO4 and concentrated. The crude product obtained was purified by chromatography on silica gel using EtOAc/hexane mixtures of increasing polarity as eluent, to give 2.08 g of the title compound of the example as an oil (77% yield). 1H-NMR (300 MHz, CDCl3 δ TMS): 1.39 (d, J=6 Hz, 6H), 4.67 (m, 1H), 6.96 (d, J=8.7 Hz, 2H), 7.81 (d, J=8.7 Hz, 2H), 9.87 (s, 1H).
References:
US2003/176481,2003,A1
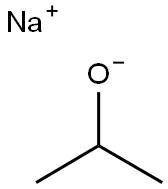
683-60-3
119 suppliers
$39.89/5g
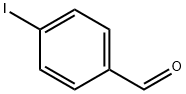
15164-44-0
294 suppliers
$6.00/1g

18962-05-5
190 suppliers
$6.00/1g

683-60-3
119 suppliers
$39.89/5g
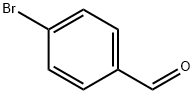
1122-91-4
702 suppliers
$9.00/10g

18962-05-5
190 suppliers
$6.00/1g