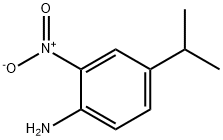
4-ISOPROPYL-2-NITROANILINE synthesis
- Product Name:4-ISOPROPYL-2-NITROANILINE
- CAS Number:63649-64-9
- Molecular formula:C9H12N2O2
- Molecular Weight:180.2
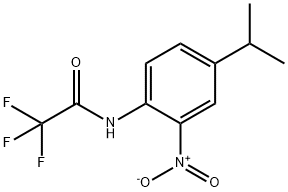
1338684-26-6

63649-64-9
The general procedure for the synthesis of 4-isopropyl-2-nitroaniline from 2,2,2-trifluoro-N-(2-nitro-4-isopropylphenyl)acetamide was as follows: to a stirring solution of 2,2,2-trifluoro-N-(4-isopropyl-2-nitrophenyl)acetamide (2.1 g, 7.6 mmol) in methanol (40 mL) was added sequentially water (20 mL) and potassium carbonate ( 0.5 g, 4 mmol). The reaction mixture was stirred at room temperature for 18 h, followed by partition extraction with ethyl acetate (50 mL) and saturated saline (50 mL). After separation of the organic layer, the aqueous layer was then extracted with ethyl acetate (3 x 25 mL). All organic layers were combined, dried with anhydrous sodium sulfate and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (silica gel 230-400 mesh, 150 g, eluting sequentially with 20% and 30% ethyl acetate/hexane solvent mixture) to afford 4-isopropyl-2-nitroaniline 1.18 g (61% yield) as an orange oil. Its NMR hydrogen spectrum (CDCl3) data were as follows: δ 1.23 (6H, d), 2.85 (1H, m), 6.77 (1H, d), 7.28 (1H, dd), 7.96 (1H, d). Mass spectrometry (ESI+) showed the molecular ion peak m/z 181.2 ([M+H]+). The HPLC (Method D) retention time was 3.97 min.

1338684-26-6
3 suppliers
inquiry

63649-64-9
65 suppliers
$37.00/250mg
Yield:63649-64-9 61%
Reaction Conditions:
with water;potassium carbonate in methanol at 20; for 18 h;
Steps:
2
To a well-stirred solution of 2,2,2-trifluoro-N-(4-isopropyl-2-nitrophenyl)acetamide (2.1 g, 7.6 mmol) in MeOH (40 mL) is added water (20 mL) and potassium carbonate (0.5 g, 4 mmol). The reaction mixture is stirred at rt for 18 h and partitioned between ethyl acetate and brine (50 mL each). The layers are separated and the aqueous layer is extracted with ethyl acetate (3x 25 mL). The organic layers are combined, dried with anhydrous sodium sulfate and concentrated. The residue is subjected to silica gel chromatography (230-400 mesh, 150 g, elution with 20 and 30% ethyl acetate/hexane) to give 1.18 g (61%) of 4- isopropyl-2-nitroaniline as an orange oil. NMR (CDCI3) δ 1.23 (6 H, d), 2.85 (1 H, hep), 6.77 (1 H, d), 7.28 (1 H, dd), 7.96 (1 H, d); MS (ESI+) for C9H12N202 m/z 181.2 (M+H)+; HPLC retention time: 3.97 min. (Method D).
References:
WO2011/126567,2011,A1 Location in patent:Page/Page column 187
40655-36-5
12 suppliers
$126.00/500mg

63649-64-9
65 suppliers
$37.00/250mg
99-88-7
231 suppliers
$8.00/5g

63649-64-9
65 suppliers
$37.00/250mg
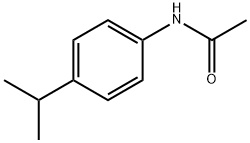
5702-74-9
50 suppliers
$15.00/1g

63649-64-9
65 suppliers
$37.00/250mg