
4-Isopropyl-2-nitrobenzoic acid synthesis
- Product Name:4-Isopropyl-2-nitrobenzoic acid
- CAS Number:35480-95-6
- Molecular formula:C10H11NO4
- Molecular Weight:209.2
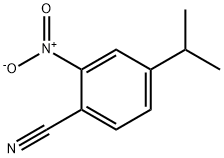
204850-15-7
10 suppliers
inquiry

35480-95-6
12 suppliers
inquiry
Yield: 73%
Reaction Conditions:
Stage #1:4-isopropyl-2-nitrobenzonitrile with sulfuric acid;water in acetic acid for 72 h;Heating / reflux;
Stage #2: with sodium hydroxide in water;acetic acid; pH=12 at 0;
Stage #3: with hydrogenchloride in water; pH=2
Steps:
91.91C
The product of Example 91B (1.746 g, 9.1798 mmol) dissolved in a 2: 1 : 1 v/v/v mixture of water/acetic acid/concentrated sulfuric acid (24 mL) was heated at reflux for 3 days. The reaction was cooled, poured onto ice water (80 mL) and adjusted to pH12 with 6N aqueous sodium hydroxide. The reaction was washed with ethyl ether (3x50 mL). The aqueous phase was acidified to pH 2 with concentrated hydrochloric acid and extracted with ethyl ether (2x75mL). The ethereal extracts were dried over magnesium sulfate, filtered, and concentrated by rotary evaporation. The residue was co-concentrated with methylene chloride (5 mL)/hexanes (100 mL) three times and dried
References:
ABBOTT LABORATORIES WO2008/133753, 2008, A2 Location in patent:Page/Page column 129-130
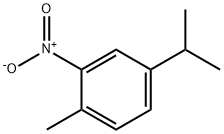
943-15-7
97 suppliers
$34.00/5g

35480-95-6
12 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

35480-95-6
12 suppliers
inquiry
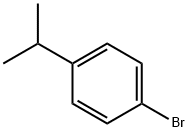
586-61-8
181 suppliers
$9.00/1g

35480-95-6
12 suppliers
inquiry
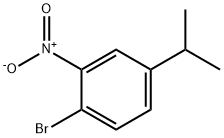
204850-14-6
8 suppliers
inquiry

35480-95-6
12 suppliers
inquiry