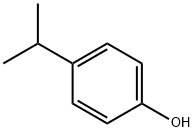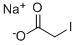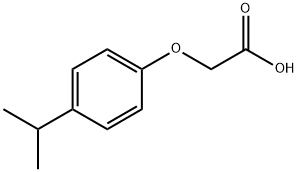
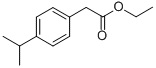
4-ISOPROPYLPHENOXYACETIC ACID synthesis
- Product Name:4-ISOPROPYLPHENOXYACETIC ACID
- CAS Number:1643-16-9
- Molecular formula:C11H14O3
- Molecular Weight:194.23
14062-21-6

1643-16-9
1. 4-Isopropylphenol (1.007 g, 7.39 mmol) was dissolved in 15 mL of dimethylformamide as starting material, potassium carbonate (2.04 g, 14.79 mmol) and ethyl bromoacetate (1.23 mL, 11.09 mmol) were added. The reaction mixture was stirred at room temperature for 48 hours. After completion of the reaction, the mixture was diluted with 500 mL of water and extracted with ether (2 x 200 mL). The organic phases were combined, dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The resulting crude product was purified by fast column chromatography (eluent: 10% ethyl acetate/hexane) to give 1.61 g (98%) of ethyl 4-isopropylphenoxyacetate as a colorless oil.MS (APCI) m/z: 223.3 [M + H]+. 1H NMR (400 MHz, CDCl3) δ: 7.14 (d, 2H), 6.84 (d, 2H) , 4.59 (s, 2H), 4.27 (q, 2H), 2.86 (m, 1H), 1.30 (t, 3H), 1.21 (d, 6H). 2. A mixture of ethyl 4-isopropylphenoxyacetate (1.61 g, 7.24 mmol) and 2 N NaOH aqueous solution (10.9 mL) in 20 mL of methanol was stirred at room temperature for 3 hr and then concentrated under reduced pressure. The residue was dissolved in 100 mL of water, acidified with 1 N aqueous hydrochloric acid to pH < 7, and then extracted with ethyl acetate (2 x 100 mL). The organic phases were combined, dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure to give 1.32 g (94%) of 4-isopropylphenoxyacetic acid as a white solid.MS (APCI) m/z: 195.3 [M + H]+.1H NMR (400 MHz, CDCl3) δ: 7.17 (d, 2H), 6.86 (d, 2H), 4.66 (s, 2H) , 2.87 (m, 1H), 1.22 (d, 6H). 3. Benzyl 2-methyl-2-(3-piperidin-3-yl-phenoxy)-propionate (30 mg, 0.085 mmol) was dissolved in 1 mL of dichloromethane, 4-isopropylphenoxyacetic acid (33 mg, 0.17 mmol) and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (33 mg, 0.17 mmol) were added and stirred at room temperature for 18 hours. The reaction mixture was concentrated under reduced pressure and the crude product was purified by fast column chromatography (eluent: 30% ethyl acetate/hexane) to afford 35 mg (78%) of benzyl 2-(3-{1-{(4-isopropylphenoxy)acetyl]piperidin-3-yl}-phenoxy)-2-methyl-propanoate as a colorless oil.LC-MS m/z: 530.6 [M + H]+. 1H NMR (400 MHz, CDCl3) δ: 7.24 (m, 5H), 7.14 (m, 3H), 6.89 (m, 2H), 6.83 (m, 1H), 6.71 (s, 1H), 6.61 (d, 1H), 5.19 (s, 2H), 4.64 (m, 3H), 4.07 (d, 1H), 3.04 (t, 1H), 2.97 (m, 1H), 2.89 (m, 1H), 2.47 (m, 2H), 1.95 (m, 1H), 1.82 (m, 1H), 1.61 (s, 6H), 1.21 (d, 6H). 4. Benzyl 2-(3-{1-[(4-isopropylphenoxy)acetyl]piperidin-3-yl}-phenoxy)-2-methyl-propanoate (35 mg, 0.066 mmol) was dissolved in 2 mL of methanol, 10% palladium-carbon (4 mg, 10 wt%) was added, and hydrogenated at atmospheric pressure for 3 hours. The reaction mixture was filtered through diatomaceous earth and washed well with ethyl acetate. The filtrates were combined and concentrated under reduced pressure to give 29 mg (99%) of 2-(3-{1-[(4-isopropylphenoxy)acetyl]piperidin-3-yl}-phenoxy)-2-methyl-propanoic acid as a colorless oil.LC-MS m/z: 440.5 [M + H]+. 1H NMR (400 MHz, CDCl3) δ: 7.19 (t, 1H), 7.14 (t, 2H), 6.87 (m, 3H), 6.81 (m, 2H), 4.66 (m, 3H), 4.04 (dd, 1H), 3.05 (m, 1H), 2.85 (m, 1H), 2.65 (m, 2H), 2.02 (t, 1H), 1.82 (t, 1H), 1.65 (m, 1H), 1.59 (s, 6H), 1.21 (d, 6H). 1.21 (d, 6H).

14062-21-6
8 suppliers
inquiry

1643-16-9
90 suppliers
$9.00/1g
Yield: 94%
Reaction Conditions:
Stage #1:(4-isopropylphenyl)acetic acid ethyl ester with sodium hydroxide;water in methanol at 20; for 3 h;
Stage #2: with hydrogenchloride in methanol;water
Steps:
2 Example 2; 2-(3-{1-[(4-Isopropyl-phenoxy)-acetyl]-piperidin-3-yl}-phenoxy)-2-methyl-propionic acid
To a solution of 4-isopropylphenol (1. 007g, 7.39 mmol) in 15 mL DIMETHYLFORMAMIDE was added potassium carbonate (2.04g, 14.79 mmol) and ethyl bromoacetate (1.23 mL, 11.09 MMOL). The reaction was stirred for 48 h at ambient temperature. The mixture was diluted with 500 mL water and extracted with diethyl ether (2 x 200 mL). The organic extracts were combined, dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The resultant oil was flash chromatographed with 10% ethyl acetate/hexanes to yield 1. 61G (98%) of ETHYL- (4-ISOPROPYLPHENOXY) acetate as a clear oil. MS (APCI) 223.3 (M + H) +. 'H NMR (400 MHz, CDCI3) 8 7.14 (d, 2H), 6.84 (d, 2H), 4.59 (s, 2H), 4.27 (q, 2H), 2.86 (m, 1H), 1.30 (t, 3H), 1.21 (d, 6H). A mixture of ETHYL- (4-ISOPROPYLPHENOXY) acetate (1.61g, 7.24 mmol) and 2N NaOH (aq) (10.9 mL) in 20 mL of methanol was stirred at ambient temperature for 3 h and concentrated under reduced pressure. The resulting residue was taken up in water (100 mL), acidified with 1N aqueous hydrochloric acid and extracted with ethyl acetate (2 x 100 mL). The organic extracts were combined, dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure to yield 1. 32G (94%) of 4-isopropylphenoxyacetic acid as a white solid. MS (APCI) 195.3 (M + H) +. 'H NMR (400 MHz, CDCI3) 8 7.17 (d, 2H), 6.86 (d, 2H), 4.66 (s, 2H), 2.87 (m), 1 H), 1.22 (d, 6H). To a solution of 2-METHYL-2- (3-PIPERIDIN-3-YL-PHENOXY)-PROPIONIC acid benzyl ester (Preparation 2, Method C; 30 mg, 0.085 mmol) in 1 mL methylene chloride was added 4-isopropylphenoxyacetic acid (33mg, 0.17 mmol) and 1- (3- DIMETHYLAMINOPROPYL)-3-ETHYLCARBODIIMIDE HYDROCHLORIDE (33mg, 0.17 mmol) and allowed to stir 18 h at ambient temperature. The reaction was concentrated under reduced pressure and the resultant oil flash chromatographed with 30% ethyl acetate/hexanes to yield 35mg (78%) OF 2- (3- {1- [ (4-ISOPROPYL-PHENOXY)-ACETYL]- PIPERIDIN-3-YL}-PHENOXY)-2-METHYL-PROPIONIC acid benzyl ester as a clear oil. LC-MS 530.6 (M + H) +. 'H NMR (400 MHz, CDCI3) S 7.24 (m, 5H), 7.14 (m, 3H), 6.89 (m, 2H), 6.83 (m, 1H), 6.71 (s, 1H), 6.61 (d, 1H), 5.19 (s, 2H), 4.64 (m, 3H), 4.07 (d, 1H), 3.04 (t, 1 H), 2.97 (m, 1 H), 2.89 (m, 1 H), 2.47 (m, 2H), 1.95 (m, 1 H), 1.82 (m, 1 H), 1.61, (s, 6H), 1.21 (d, 6H). 10% Palladium on carbon (4 mg, 10 wt%) was added to a solution of 2- (3- {1- [ (4-ISOPROPYL-PHENOXY)-ACETYL]-PIPERIDIN-3-YLL-PHENOXY)-2-METHYL-PROPIONIC ACID BENZYI ester (35 mg, 0.066 mmol) in methanol (2 mL) and the resulting mixture hydrogenated at atmospheric pressure for 3 h. The reaction mixture was filtered through a plug of celite and the celite plug washed thoroughly with ethyl acetate. The combined filtrates were concentrated under reduced pressure to provide 29 mg (99%) of 2-(3-{1-[(4-Isopropyl-phenoxy)-acetyl]-piperidin-3-yl]-phenoxy)-2-methyl- propionic acid as a clear oil. LC-MS 440.5 (M + H) +. 'H NMR (400 MHz, CDCI3) 8 7.19 (t, 1H), 7.14 (t, 2H), 6.87 (m, 3H), 6.81 (m, 2H), 4.66 (m, 3H), 4.04 (dd, 1 H), 3.05 (m, 1H), 2.85 (m, 1 H), 2.65 (m, 2H), 2.02 (t, 1H), 1.82 (t, 1H), 1.65 (m, 1H), 1.59, (s, 6H), 1.21 (d, 6H).
References:
PFIZER PRODUCTS INC. WO2004/48334, 2004, A1 Location in patent:Page 128-130
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

1643-16-9
90 suppliers
$9.00/1g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

1643-16-9
90 suppliers
$9.00/1g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

1643-16-9
90 suppliers
$9.00/1g