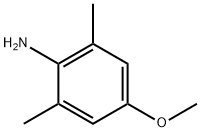
4-methoxy-2,6-dimethyl-aniline synthesis
- Product Name:4-methoxy-2,6-dimethyl-aniline
- CAS Number:34743-49-2
- Molecular formula:C9H13NO
- Molecular Weight:151.21
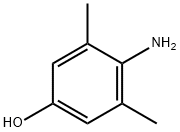
3096-70-6

74-88-4

34743-49-2
Under argon protection, 4-amino-3,5-dimethylphenol (50 mg, 0.36 mmol) and sodium 2-methylpropan-2-ol (52 mg, 0.55 mmol) were dissolved in anhydrous DMF (1 mL). Iodomethane (0.021 mL, 0.33 mmol) was then added and the reaction mixture was stirred overnight at room temperature. After the reaction was completed, dichloromethane (20 mL) was added to dilute the reaction and washed sequentially with aqueous sodium hydroxide (2 × 15 mL) and saturated saline (2 × 15 mL). The organic layer was dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (eluent: hexane/ethyl acetate=2/1) to afford 4-methoxy-2,6-dimethylaniline (24 mg, 43% yield). Mass spectrum (ESI+) m/z: 152.1 [M+H]+. Methyl 7-[3-(N-(4-methoxy-2,6-dimethylphenyl)aminosulfonyl)phenyl]heptanoate (12 mg, 17% yield) was synthesized by following the procedure described in Method A using 4-methoxy-2,6-dimethylaniline (24 mg, 0.16 mmol) as starting material. Mass spectrum (ESI+) m/z: 456.2 [M+Na]+. The above ester derivative (12 mg, 0.028 mmol) was hydrolyzed according to the hydrolysis conditions described in Method A to give compound 8 as an orange oil (11 mg, 95% yield).1H NMR (300 MHz, chloroform-d) δ ppm: 1.22-1.36 (m, 4H), 1.49-1.66 (m, 4H), 1.97 (s, 6H) , 2.30 (t, J=6.76 Hz, 2H), 2.61 (t, J=7.58 Hz, 2H), 3.75 (s, 3H), 6.53 (s, 1H), 7.31-7.39 (m, 3H), 7.46-7.57 (m, 3H). Mass spectrum (ESI+) m/z: 442.2 [M+Na]+.
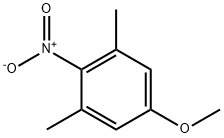
61019-03-2
49 suppliers
inquiry

34743-49-2
82 suppliers
$26.00/100mg
Yield: 78%
Reaction Conditions:
Stage #1:5-methoxy-1,3-dimethyl-2-nitrobenzene with tin(ll) chloride in ethanolHeating / reflux;
Stage #2: with sodium hydroxide;water
Steps:
A.A14.b Example A14; b) Preparation of intermediate 35
To a solution of intermediate 34 (1.81 g, 10 mmoles) in ethanol (20 ml) was added tin (II) chloride dihydrate (11.51 g, 50 mmoles) and the mixture was refluxed overnight. Upon cooling, ice was added to the reaction mixture followed by basification with 2N NaOH. The mixture was filtered and the filtrate was concentrated under reduced pressure. The aqueous solution was extracted with dichloromethane (4 x 30 ml). The organic layers were combined and dried over anhydrous MGSO4 and the solvent was removed under reduced pressure. The residue was purified on a silica gel column chromatography (CH2CL2 as eluent). Yield: 1.18 g of intermediate 35 (78%).
References:
JANSSEN PHARMACEUTICA N.V.;ARTS, Theodora, Joanna, Francisca;JANSSEN, Graziella, Maria, Constantina;JANSSEN, Herwig, Josephus, Margareta;JANSSEN, Jasmine, Josée, Werner;JANSSEN, Paul, Peter, Maria;JANSSEN, Maroussia, Godelieve, Frank WO2004/74266, 2004, A1 Location in patent:Page 54-55

3096-70-6
143 suppliers
$20.00/1g

74-88-4
356 suppliers
$15.00/10g

34743-49-2
82 suppliers
$26.00/100mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

34743-49-2
82 suppliers
$26.00/100mg

6981-15-3
12 suppliers
inquiry

34743-49-2
82 suppliers
$26.00/100mg
37934-89-7
72 suppliers
$45.00/1g

34743-49-2
82 suppliers
$26.00/100mg