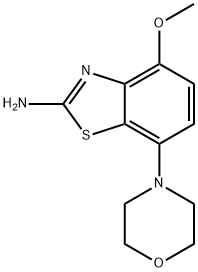
4-Methoxy-7-Morpholinobenzo[d]thiazol-2-aMine synthesis
- Product Name:4-Methoxy-7-Morpholinobenzo[d]thiazol-2-aMine
- CAS Number:383865-57-4
- Molecular formula:C12H15N3O2S
- Molecular Weight:265.33
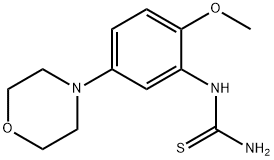
383870-59-5
![4-Methoxy-7-Morpholinobenzo[d]thiazol-2-aMine](/CAS/20150408/GIF/383865-57-4.gif)
383865-57-4
The general procedure for the synthesis of 4-methoxy-7-N-morpholino-benzo[D]thiazol-2-amine using 1-(2-methoxy-5-morpholinophenyl)thiourea as starting material was as follows: 1-(2-methoxy-5-morpholinophenyl)thiourea (5.0 g, 19 mmol) was dissolved in chloroform (130 ml), and bromine (960 μl) was added slowly, followed by heating and refluxing the reaction mixture for for 18 hours. After completion of the reaction, the volatile solvent was removed by distillation under reduced pressure and the resulting crude product was purified by recrystallization from tetrahydrofuran (THF) to afford the target compound 4-methoxy-7-N-morpholino-benzo[D]thiazol-2-amine (2.8 g, 57% yield). The result of mass spectrometry analysis: m/e = 266 (M+).

383870-59-5
25 suppliers
inquiry
![4-Methoxy-7-Morpholinobenzo[d]thiazol-2-aMine](/CAS/20150408/GIF/383865-57-4.gif)
383865-57-4
43 suppliers
$46.00/100mg
Yield:383865-57-4 95%
Reaction Conditions:
Stage #1: (2-methoxy-5-morpholin-4-yl-phenyl)thioureawith hydrogen bromide;acetic acid in ethyl acetate at 80 - 100; for 0.2 h;
Stage #2: with dimethyl sulfoxide in ethyl acetate; for 4 h;Heating;Further stages;
Steps:
4.5.6. 4-Methoxy-7-morpholin-4-yl-benzothiazol-2-ylamine 7
Under efficient mechanical stirring the thiourea 6 (6.05 g,22.6 mmol) was suspended in ethyl acetate (67 mL) and heated to80 C. At that temperature 33% HBr in acetic acid (8.18 mL,45.2 mmol) was added dropwise over 0.2 h. After complete additionthe thick suspension was refluxed (oil bath 100 C) for 0.2 hand DMSO (1.93 mL, 27.12 mmol) was added at that temperature inone portion. Ten minutes after the addition ethyl acetate (20 mL)was added and stirring and heating was continued for 4 h. Themixture was cooled to ambient temperature, the solid collected byfiltration and washed with ethyl acetate (50 mL). The wet materialwas suspended under stirring in EtOH (55 mL), water (72 mL) wasadded, and the red solution thus formed was heated to 50 C.Aqueous ammonia (28%, 5.4 mL) was added (pH 9e10), the mixturewas cooled in an ice bath, and stirred overnight (during this timethe ice bath was allowed to thaw). The light brown precipitateformed was collected by filtration and washed with 50% aqueousethanol (TLC: ethyl acetate/methanol, 90/10). The solid wasextracted with boiling THF (100 mL), cooled, collected by filtration,and oven-dried at 100 C to give the aminobenzothiazole as greycrystals, 5.7 g (95% yield), mp > 275 C (dec) (TLC: ethyl acetate/methanol/AcOH, 90/10/0.2, v/v/v). If further purification wasneeded the solid was dissolved in aqueous 0.5 N HCl (50 mL,25 mmol) and stirred for 0.2 h at ambient temperature. Insolublematerial was removed by filtration, aqueous NH4OH (28%, 14.47 M,2.07 mL, 30 mmol) was added, and the formed suspension wasstirred for 0.2 h. EtOH (75 mL) was added and the mixture waschilled overnight. The solid was collected by filtration, washed with50% aqueous ethanol and vacuum-dried in a desiccator overP4O10.1H NMR (400 MHz, DMSO-d6) δ 2.94 (m, 4H, 2 x N-CH2), 3.74(m, 4H, 2 x O-CH2), 3.81 (s, 3H, O-CH3), 6.64 (d, 1H, J 8.7 Hz, H5),6.78 (d, 1H, J 8.7 Hz, H6), 7.42 (sbr, 2H, NH2). 13C NMR (101 MHz,DMSO-d6) δ 51.7 (N-CH2), 56.6 (O-CH3), 67. (O-CH2), 109.4 (C6), 110.7(C5), 126.1 (C7a), 140.4 (C4), 143.4 (C7), 146.9 (C4a) 165.8 (C2).C12H15N3O2S MS (ESI) m/z: [MH] Calcd 266.09; Found 266.17.
References:
Renk, Dana R.;Skraban, Marcel;Bier, Dirk;Schulze, Annette;Wabbals, Erika;Wedekind, Franziska;Neumaier, Felix;Neumaier, Bernd;Holschbach, Marcus [European Journal of Medicinal Chemistry,2021,vol. 214]
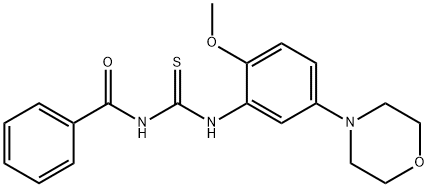
383870-86-8
20 suppliers
inquiry
![4-Methoxy-7-Morpholinobenzo[d]thiazol-2-aMine](/CAS/20150408/GIF/383865-57-4.gif)
383865-57-4
43 suppliers
$46.00/100mg
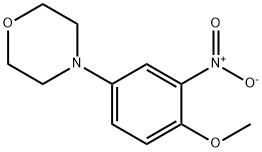
383870-96-0
19 suppliers
inquiry
![4-Methoxy-7-Morpholinobenzo[d]thiazol-2-aMine](/CAS/20150408/GIF/383865-57-4.gif)
383865-57-4
43 suppliers
$46.00/100mg
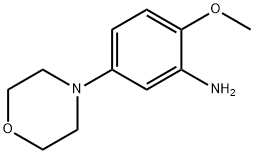
383870-88-0
30 suppliers
inquiry
![4-Methoxy-7-Morpholinobenzo[d]thiazol-2-aMine](/CAS/20150408/GIF/383865-57-4.gif)
383865-57-4
43 suppliers
$46.00/100mg
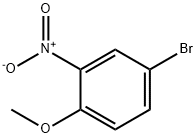
33696-00-3
206 suppliers
$10.00/1g
![4-Methoxy-7-Morpholinobenzo[d]thiazol-2-aMine](/CAS/20150408/GIF/383865-57-4.gif)
383865-57-4
43 suppliers
$46.00/100mg