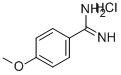
4-METHOXYBENZAMIDINE, HYDROCHLORIDE synthesis
- Product Name:4-METHOXYBENZAMIDINE, HYDROCHLORIDE
- CAS Number:51721-68-7
- Molecular formula:C8H11ClN2O
- Molecular Weight:186.64
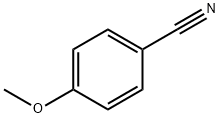
874-90-8

51721-68-7
General procedure for the synthesis of 4-methoxybenzenecarbamidine hydrochloride from 4-methoxybenzyl cyanide: 4-methoxybenzyl cyanide (2.1 g, 15.7 mmol) was mixed with sodium methanolate (86 mg, 1.57 mmol) in 20 mL of anhydrous methanol, and the reaction was stirred for 48 hours at room temperature. Subsequently, ammonium chloride (0.84 g, 15.7 mmol) was added to the reaction system and stirring was continued for 24 hours. After completion of the reaction, the precipitate was collected by filtration, washed with ether and dried under vacuum to afford the target product 4-methoxybenzenecarboximidamide hydrochloride (1.3 g, 44.5% yield). Mass spectrum (ESI) m/z: 151.1 ([M+H]+).

874-90-8
335 suppliers
$8.00/10g

51721-68-7
98 suppliers
$12.00/250mg
Yield: 44.5%
Reaction Conditions:
Stage #1:4-methoxybenzonitrile with sodium methylate in methanol at 20; for 48 h;
Stage #2: with ammonium chloride in methanol at 20; for 24 h;
Steps:
3 4.1.3. Synthesis of 4-methoxy-benzamidine hydrochloride 3
A solution of 4-methoxybenzonitrile 2 (2.1 g, 15.7 mmol), sodium methylate (86 mg, 1.57 mmol) in 20 mL of anhydrous methanol was stirred at room temperature for 48 h. Then ammonium chloride (0.84 g, 15.7 mmol) was added, and the reaction group was stirred for another 24 h the precipitate was collected by filtration, washed with diethyl ether, and then dried in vacuum to obtain 3 as a white solid (1.3 g, 44.5%), MS (ESI) m/z: 151.1 ([MH]).
References:
Luo, Guoshun;Tang, Zhichao;Lao, Kejing;Li, Xinyu;You, Qidong;Xiang, Hua [European Journal of Medicinal Chemistry,2018,vol. 150,p. 783 - 795]
54998-29-7
25 suppliers
$175.00/1g

51721-68-7
98 suppliers
$12.00/250mg

39739-49-6
3 suppliers
inquiry

51721-68-7
98 suppliers
$12.00/250mg

4222-26-8
1 suppliers
inquiry

51721-68-7
98 suppliers
$12.00/250mg
123-11-5
885 suppliers
$10.00/10g

51721-68-7
98 suppliers
$12.00/250mg