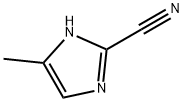
4-Methyl-1H-iMidazole-2-carbonitrile synthesis
- Product Name:4-Methyl-1H-iMidazole-2-carbonitrile
- CAS Number:70631-95-7
- Molecular formula:C5H5N3
- Molecular Weight:107.11
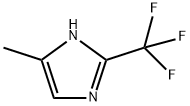
66675-23-8

70631-95-7
4-Methyl-2-(trifluoromethyl)-1H-imidazole (Compound 14.1, 800 mg, 5.33 mmol) was added to a 250 mL round-bottomed flask under inert nitrogen protection. Subsequently 5% aqueous ammonium hydroxide solution (50 mL) was added. The reaction mixture was stirred at 25-30°C for 40 hours. Upon completion of the reaction, the pH of the reaction solution was adjusted with acetic acid to 5-6. The aqueous phase was extracted with ethyl acetate (3 x 150 mL), and the organic phases were combined and washed with brine (2 x 50 mL) and saturated aqueous Na2CO3 solution (2 x 20 mL) in that order. The organic layer was dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure. The crude product was purified by silica gel column chromatography with the eluent of ethyl acetate: petroleum ether (1:20-1:10) to afford 4-methyl-1H-imidazole-2-carbonitrile (350 mg, 61% yield) as a white solid.

66675-23-8
5 suppliers
inquiry

70631-95-7
25 suppliers
inquiry
Yield:70631-95-7 61%
Reaction Conditions:
with ammonium hydroxide at 25 - 30; for 40 h;Inert atmosphere;
Steps:
1 Compound 14.2. 4-Methyl-lH-imidazole-2-carbonitrile
Compound 14.2. 4-Methyl-lH-imidazole-2-carbonitrile. Into a 250-mL round- bottom flask, which was purged and maintained with an inert atmosphere of nitrogen, was placed 4-methyl-2-(trifluoromethyl)-lH-imidazole (compound 14.1, 800 mg, 5.33 mmol) and 5% aqueous ammonium hydroxide (50 mL). The resulting solution was stirred for 40 h at 25- 30 °C, then the pH of the solution was adjusted to 5-6 with acetic acid. The aqueous phase was extracted with ethyl acetate (3 x 150 mL) and the combined organic extracts were washed with brine (2 x 50 mL) and aqueous Na2C03 (sat., 2 x 20 mL), dried (Na2SC>4), filtered, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography with ethyl acetate:PE (1 :20-1 : 10) as the eluent to yield 350 mg (61 %) of the title compound as a white solid.
References:
WO2014/8197,2014,A1 Location in patent:Page/Page column 87; 88