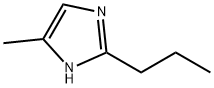
4-Methyl-2-propyl-1H-imidazole synthesis
- Product Name:4-Methyl-2-propyl-1H-imidazole
- CAS Number:37455-55-3
- Molecular formula:C7H12N2
- Molecular Weight:124.18

123-72-8
438 suppliers
$12.32/25ML
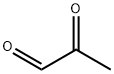
78-98-8
307 suppliers
$10.60/1gm:

37455-55-3
27 suppliers
inquiry
Yield:37455-55-3 65%
Reaction Conditions:
with ammonium hydroxide in ethanol; for 12 h;
Steps:
1.6 1.6 4-methyl-2-propyl- 1 H-imidazo le
1.6 4-methyl-2-propyl- 1 H-imidazo le A mixture of butyraldehyde (5.0 g, 69.3 mmol) and ammonium hydroxide (15 mL, 385 mmol) in ethanol (15 mL) was stirred at 60 °C in a 50 mL round-bottomed flask. A solution of 2-oxopropanal (25.0 g, 104 mmol) was added. The mixture was stirred for 12 h. The reaction mixture was diluted with water. The aqueous layer was extracted with ethyl acetate (3 x 50 mL). The combined organic layers were washed with brine, dried with Na2S04, filtered and concentrated to afford a yellow solution. The resulting mixture was deposited onto silica gel and loaded onto a silica gel column and eluted with PE/EA(1 : 1) to give the title compound (5.6 g, 45.1 mmol, 65% ) as a brown liquid. LC-MS: m/z 125 (M+H), RT=1.39 min; 1H NMR (400 MHz, CDC13): δ =10.46 (s, 1H), 8.60 (s, 1H), 2.67 (t, J = 8.0 Hz, 2H), 2.20 (s, 3 H), 1.74-1.69 (m, 2H), 0.92 (t, J = 7.2 Hz, 3H).
References:
WO2014/41175,2014,A1 Location in patent:Page/Page column 56

123-72-8
438 suppliers
$12.32/25ML
116-09-6
266 suppliers
$11.00/25g

37455-55-3
27 suppliers
inquiry