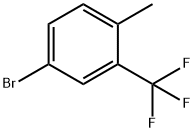
4-METHYL-3-(TRIFLUOROMETHYL)BROMOBENZENE synthesis
- Product Name:4-METHYL-3-(TRIFLUOROMETHYL)BROMOBENZENE
- CAS Number:86845-27-4
- Molecular formula:C8H6BrF3
- Molecular Weight:239.03
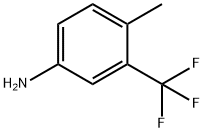
65934-74-9

86845-27-4
Under nitrogen protection, 2642.3 g of a 48% aqueous solution of hydrogen bromide was added to the reactor. The temperature of the system was adjusted to 30 °C, followed by the slow dropwise addition of 1760.0 g of a mixed solution of water and 880.0 g of acetic acid while stirring. Next, 880.0 g of 2-methyl-5-aminobenzotrifluoride (isomer content 0.037%, determined by gas phase area normalization) was added. After the dropwise addition, the reaction temperature was raised to 95 °C with continuous stirring for 30 min to obtain the corresponding hydrogen bromide salt solution. The reaction solution was cooled to 0 °C, at which temperature 1213.3 g of a 30% aqueous sodium nitrite solution was slowly added dropwise. After completion of the dropwise addition, the reaction was continued for 1.0 hour to ensure complete reaction. The prepared diazonium salt solution was slowly added dropwise at 65 °C to a pre-prepared mixture containing 360.4 g of copper bromide and 4997.0 g of 48% aqueous hydrogen bromide. A large amount of nitrogen release was observed during the reaction. After dropwise addition, stirring was continued for 1 hour to obtain a solution of 4-bromo-1-methyl-2-(trifluoromethyl)benzene. At the end of the reaction, the reaction solution was cooled to 20 °C, stirred for 1.0 h and left to stratify. 1046.0 g of the organic layer was separated and 3661.0 g of water was added and the pH was adjusted with 5.0% alkali metal hydroxide solution to 7. Subsequent steam distillation afforded 1032.0 g of a pale yellow 4-bromo-1-methyl-2-(trifluoromethyl)benzene product in 85.9% yield and 99.2% gas phase purity. Gas chromatographic analysis showed that the isomer content in the product was less than 0.1%.

65934-74-9
212 suppliers
$9.00/1g

86845-27-4
122 suppliers
$11.00/1g
Yield: 85.9%
Reaction Conditions:
Stage #1:3-(trifluoromethyl)-4-methylaniline with hydrogen bromide;acetic acid in water at 30 - 95; for 0.5 h;Inert atmosphere;Large scale;
Stage #2: with sodium nitrite in water at 0; for 1 h;Inert atmosphere;Large scale;
Stage #3: with hydrogen bromide;copper(I) bromide in water at 65; for 1 h;Large scale;Concentration;Temperature;
Steps:
2 Example 2
Under nitrogen protection, Add 2642.3 g of 48% aqueous hydrogen bromide solution at room temperature. 1760.0g water and 880.0g acetic acid, Stirring, 880.0 g of 2-methyl-5-amino-benzotrifluoride (isoforms 0.037%, gas phase area normalized) were added dropwise at 30°C. After dropping, The temperature was raised to 95°C and stirred for 30 minutes to obtain a solution of hydrogen bromide salt.Cooling to 0°C, Add dropwise 1213.3g of 30% aqueous sodium nitrite solution at 0°C. The reaction was completed 1.0 h after the addition. The prepared diazonium salt was added dropwise to a previously prepared 360.4 g of cuprous bromide and 4997.0 g of a 48% aqueous solution of hydrogen bromide at 65C. A lot of nitrogen is released, Stirring is completed 1h, A 4-bromo-1-methyl-2-(trifluoromethyl)benzene solution was obtained.After the reaction is over, Cool down to 20°C and stir for 1.0h Resting, Layered, 1046.0g oil layer was added to 3661.0g water, 5.0% alkali hydroxide solution is alkalized to PH=7. After vapor distillation, 1032.0 g of pale yellow 4-bromo-1-methyl-2-(trifluoromethyl)benzene product was obtained. Yield 85.9%, The gas purity is 99.2%. Gas chromatography showed that the isomer content was less than 0.1%.
References:
Jinkai (Liaoning) Chemical Co., Ltd.;Wang Yanming;Wang Yongcan;Qin Long;Song Tongji;Chen Biwei CN107915570, 2018, A Location in patent:Paragraph 0057-0061; 0064-0067