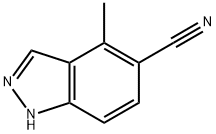
4-METHYL-5-CYANO-(1H)INDAZOLE synthesis
- Product Name:4-METHYL-5-CYANO-(1H)INDAZOLE
- CAS Number:478837-29-5
- Molecular formula:C9H7N3
- Molecular Weight:157.17
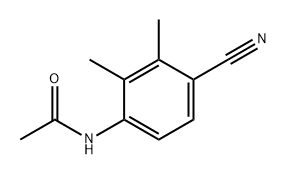
478837-27-3
0 suppliers
inquiry

478837-29-5
21 suppliers
inquiry
Yield:478837-29-5 84.9%
Reaction Conditions:
with potassium acetate;acetic anhydride;N-butylammonium bromide;isopentyl nitrite in ethyl acetate; for 7 h;Heating / reflux;
Steps:
495.f Example 495; Synthesis of 4-methyl-N-tetrahydro-2H-pyran-4-yl-1H-indazole-5-carboxamide; (f) Synthesis of 4-methyl-1H-indazole-5-carbonitrile
Acetic anhydride (2.1 ml, 22.3 mmol), tetra-n-butylammonium bromide (118 mg, 0.366 mmol), potassium acetate (1.44 g, 14.7 mmol) and isoamyl nitrite (1.3 ml, 9.68 mmol) were added to an ethyl acetate suspension (15 ml) of N-(4-cyano-2,3-dimethylphenyl)acetamide (1.38 g, 7.33 mmol) at room temperature, and then the reaction was carried out for 7 hours under reflux conditions. The reaction mixture was cooled, diluted with ethyl acetate, and then washed with a saturated aqueous sodium hydrogencarbonate solution. The organic layer was washed with a saturated aqueous sodium chloride solution, dried over magnesium sulfate, and then distilled to remove the solvent, and the resulting residue was purified by a silica gel column chromatography (eluent: n-hexane/chloroform) to obtain 4-methyl-1H-indazole-5-carbonitrile (1.24 g, 84.9%).1H-NMR (DMSO-d6) δ; 2.81 (3H, s), 2.84 (3H, s), 7.72 (1H, d, J=8.8Hz), 8.23 (1H, d, J=0.7Hz), 8.37 (1H, d, J=8.8Hz).
References:
EP1403255,2004,A1 Location in patent:Page 128
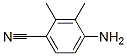
478837-24-0
2 suppliers
inquiry

478837-29-5
21 suppliers
inquiry
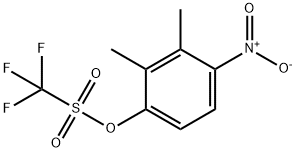
478837-20-6
0 suppliers
inquiry

478837-29-5
21 suppliers
inquiry
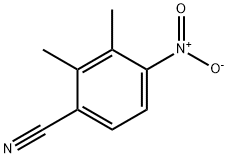
52962-97-7
5 suppliers
inquiry

478837-29-5
21 suppliers
inquiry
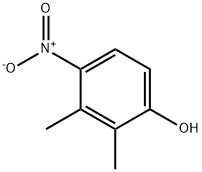
19499-93-5
88 suppliers
$12.00/250mg

478837-29-5
21 suppliers
inquiry