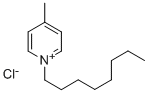
4-METHYL-N-OCTYLPYRIDINIUM CHLORIDE synthesis
- Product Name:4-METHYL-N-OCTYLPYRIDINIUM CHLORIDE
- CAS Number:141645-91-2
- Molecular formula:C14H24ClN
- Molecular Weight:241.8001
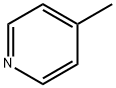
108-89-4
344 suppliers
$14.00/25mL

111-85-3
397 suppliers
$5.00/25g

141645-91-2
17 suppliers
inquiry
Yield:141645-91-2 52%
Reaction Conditions:
at 75; for 72 h;
Steps:
4-Picoline (0.4 mol) and 1-chlorobutane (or 1-chlorohexane or 1-chlorooctane) (0.4 mol) were placed into a 250 cm3 round bottom flask equipped with an electric heat-jacket and vigorously stirred using a magnetic stirrer at 75 °C for 72 h. In the case of the 1-n-butyl-4-methylpyridinium chloride, [C4-4-mpy]Cl and 1-n-hexyl-4-methylpyridinium chloride, [C6-4-mpy]Cl the brownish solid forms were obtained directly after synthesis. The resulting solids were washed in Schlenk apparatus with diethyl ether (4 × 50 cm3) and hexane (4 × 50 cm3) and dried by nitrogen stream. In the case of 1-n-octyl-4-methylpyridinium chloride, [C8-4-mpy]Cl, a transparent, brownish, dense, and viscous liquid was obtained. It was cooled to room temperature and extracted by turns with diethyl ether (4 × 50 cm3) and hexane (4 × 50 cm3). After the hexane extraction, the residual liquid crystallized. The resulting solid was filtered off by means of Schlenk apparatus and dried by nitrogen stream. Yields: 39% for [C4-4-mpy]Cl, 35% for [C6-4-mpy]Cl, and 52% for [C8-4-mpy]Cl.
References:
Och?dzan-Siod?ak, Wioletta;Dziubek, Katarzyna;Siod?ak, Dawid [Journal of Molecular Liquids,2013,vol. 177,p. 85 - 93]
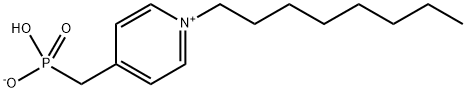
133216-93-0
0 suppliers
inquiry

141645-91-2
17 suppliers
inquiry

629-27-6
167 suppliers
$5.00/5g

141645-91-2
17 suppliers
inquiry
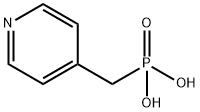
80241-43-6
15 suppliers
inquiry

141645-91-2
17 suppliers
inquiry