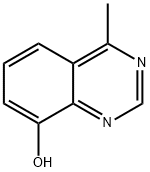
4-Methyl-quinazolin-8-ol synthesis
- Product Name:4-Methyl-quinazolin-8-ol
- CAS Number:99512-70-6
- Molecular formula:C9H8N2O
- Molecular Weight:160.17
Yield:99512-70-6 90%
Reaction Conditions:
at 180; for 0.75 h;Microwave irradiation;
Steps:
11.i Example 11 Preparation of (S)-N-methyl-8-(1-((6-(6-methylpyridin-3-yl)pyrimidin-4-yl)amino)propan-2-yl)quinazoline-4-carboxamide (Compound 971)
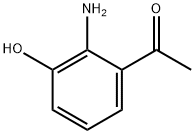
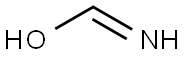
As shown in step 11-i of Scheme 11, 1-(2-amino-3-hydroxyphenyl)ethanone (4.0 g, 26.5 mmol) and formamide (20 mL, 45 mmol) were heated at 180° C. under microwave irradiation for 45 minutes.
After cooling, water was added and the reaction mixture concentrated under reduced pressure.
The residue was purified by medium pressure silica gel chromatography (2% MeOH/DCM) to produce 4-methylquinazolin-8-ol (compound 2046, 3.81 g, 90% yield) as a yellow solid.
This product was used as is in subsequent reactions.
References:
US2013/281431,2013,A1 Location in patent:Paragraph 0285
69674-28-8
6 suppliers
inquiry

99512-70-6
6 suppliers
inquiry
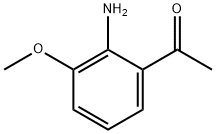
42465-54-3
46 suppliers
$98.00/100mg

99512-70-6
6 suppliers
inquiry
408507-83-5
5 suppliers
inquiry

99512-70-6
6 suppliers
inquiry