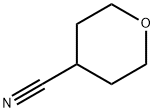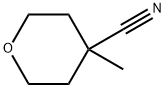
4-METHYLTETRAHYDRO-2H-PYRAN-4-CARBONITRILE synthesis
- Product Name:4-METHYLTETRAHYDRO-2H-PYRAN-4-CARBONITRILE
- CAS Number:856255-87-3
- Molecular formula:C7H11NO
- Molecular Weight:125.17
Yield:856255-87-3 87%
Reaction Conditions:
Stage #1: 4-cyanotetrahydro-4H-pyranwith lithium hexamethyldisilazane in tetrahydrofuran at 0 - 5; for 1.5 h;
Stage #2: iodomethane in tetrahydrofuran at 20; for 2 h;
Steps:
6 Synthesis of 4-methyl-4-cyanotetrahydropyran
In a 30 ml glass flask equipped with a stirrer, a thermometer and a dropping funnel, 1.0 g (9.0 mmol) of 4-cyanotetrahydropyran and 5 ml of dry tetrahydrofuran were added, and the liquid temperature was maintained at 0 to 5°C.,10.8 ml (10.8 mmol) of a 1.0 mol/l lithium bis (trimethylsilyl)amide in tetrahydrofuran solution was gently added dropwise, and the mixture was stirred at the same temperature for 1.5 hours. Then, 3.8 g (27 mmol) of iodomethane was added, the mixture was reacted at room temperature for 2 hours. After completion of the reaction, 15 mmol (15 mmol) of 1.0 mmol / l hydrochloric acid was added to the obtained reaction solution, under ice cooling, the reaction solution was concentrated. To the concentrate, 10 ml of a saturated aqueous sodium chloride solution was added, extracted twice with 30 ml of ethyl acetate, the extract was dried over anhydrous magnesium sulfate. After filtration, the concentrate was distilled under reduced pressur to obtain 0.98 g of 4-methyl-4-cyanotetrahydropyran (isolation yield: 87%) as light yellow liquid.
References:
JP5673729,2015,B2 Location in patent:Paragraph 0070
97986-34-0
56 suppliers
$28.00/100mg

856255-87-3
25 suppliers
inquiry
25637-16-5
177 suppliers
$7.00/1g

856255-87-3
25 suppliers
inquiry
134419-59-3
100 suppliers
$6.00/1g

856255-87-3
25 suppliers
inquiry