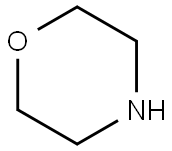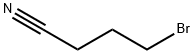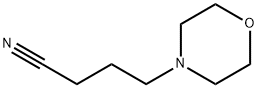
4-Morpholinobutyronitrile synthesis
- Product Name:4-Morpholinobutyronitrile
- CAS Number:5807-11-4
- Molecular formula:C8H14N2O
- Molecular Weight:154.21
Yield:5807-11-4 99%
Reaction Conditions:
with potassium carbonate in acetonitrile at 20; for 72 h;
Steps:
3.2. Preparation of -(morpholin-4-yl)nitrile Precursors
General procedure: To a mixture of morpholine, 1, (6.90 mL, 80 mmol) and anhydrous potassium carbonate (5.58 g,40 mmol) in acetonitrile (50 mL), 4-chlorobutanenitrile, 2, (1.94 mL, 20 mmol) or 5-chloropentanenitrile,3, (2.20 mL, 20 mmol) was added. The mixture was stirred for 72 h at room temperature. Afterthat time, the crude was evaporated to dryness. Then, water (50 mL) was added and the mixtureextracted with dichloromethane (3 x 30 mL). The organic extract was subsequently washed with water,dried over anhydrous Na2SO4, evaporated to dryness in a rotary evaporator, and, finally, purified byvacuum distillation to yield a yellowish oil that was identified as 3-(morpholin-4-yl)-butanenitrile(4) or 3-(morpholin-4-yl)-pentanenitrile (5), respectively [40,41]. Compound 4 was obtained in a 99%yield. 1H-NMR (CDCl3, 400 MHz): δ 3.67-3.65 (m, 4 H), 2.40-2.32 (m, 8H), 1.79 (quin, J = 6.7 Hz, 2H).13C-NMR (CDCl3, 100 MHz): δ 119.8, 67.0, 56.8, 53.6, 22.5, 14.9. Compound 5 was obtained in a 95%yield. 1H-NMR (CDCl3, 400 MHz): δ 3.67-3.65 (m, 4H), 2.40-2.32 (m, 8H), 1.71-1.58 (m, 4H). 13C-NMR(CDCl3, 100 MHz): δ 119.5, 66.7, 57.5, 53.5, 25.1, 23.2, 16.9.
References:
Savastano, Matteo;García-Gallarín, Celeste;Giorgi, Claudia;Gratteri, Paola;de la Torre, Maria Dolores López;Bazzicalupi, Carla;Bianchi, Antonio;Melguizo, Manuel [Molecules,2019,vol. 24,# 12,art. no. 2247]