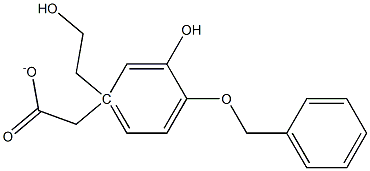
4-O-Benzyl-3-hydroxy Tyrosol α-Acetate synthesis
- Product Name:4-O-Benzyl-3-hydroxy Tyrosol α-Acetate
- CAS Number:1333081-71-2
- Molecular formula:C17H18O4
- Molecular Weight:286.32
1333081-69-8
0 suppliers
inquiry

1333081-71-2
8 suppliers
$650.00/50mg
Yield:1333081-71-2 89%
Reaction Conditions:
with methanol;ammonia at 0; for 1 h;
Steps:
To a solution of 4 (2.98 g, 15.5 mmol) in 100 ml of CH2Cl2 was added in small portions MCPBA (4.93 g, 28.6 mmol) and the mixture was stirred for 24 h at 40 °C. The solution was concentrated under reduced pressure, the residue dissolved in 60 ml of methanolic NH3 (4 M) and stirred for 72 h at rt. The mixture was concentrated and quickly filtered through a short pad of SiO2 using AcOEt/petroleum ether to give 1 (2.08 g, 87%) 1H and 13C NMR according to Lit.5e; 7: To a 0.5 M solution of 6 (3 g, 10.6 mmol) in CH2Cl2 was added a catalytic amount of diphenylselenide (180 mg) followed by the addition of 2.7 ml of 30% H2O2. The reaction mixture was stirred at rt for 48 h followed by work-up according to Syper et al.14 Flash chromatography (SiO2, CH2Cl2) gave 7 (2.66 g, 84%) as an oil. 1H NMR (200 MHz, CDCl3): δ = 8.28 (s,1H), 7.38 (m, 5H), 6.98-7.1 (m, 3H), 5.10 (s, 2H), 4.26 (t, 2H, J = 6.94 Hz), 2.89 (t, 2H, J = 6.94 Hz), 2.05 (s, 3H); 13C NMR (50 MHz, CDCl3): δ = 170.9, 159.1, 184.6, 139.0, 136.4, 131.1, 128.6, 128.1, 127.5, 127.3, 123.1, 114.2, 70.8, 64.6, 34.0, 20.9; 8: To a solution of 7 (2.64 g, 8.86 mmol) in dry AcOEt (80 ml) was added 10% Pd/C (100 mg) and hydrogenated using 1 atm of H2 (balloon) at room temperature. After 30 min the mixture was filtered through a pad of celite and washed with EtOH abs (3 × 5 ml). The filtrate was concentrated under reduced pressure to give 8 (1.83 g, 98%) as an oil. 1H NMR (200 MHz, CDCl3): δ = 8.31 (s, 1H), 6.75-7.07 (m, 3H), 4.21-4.30 (m, 2H), 2.84-2.92 (m, 2H), 2.06 (s, 3H); 13C NMR (50 MHz, CDCl3): δ = 171.42, 159.9, 146.9, 145.5, 130.7, 127.8, 122.4, 121.2, 64.8, 34.3, 21.0; 9: To a cooled (T = 0 °C) 0.1 M solution of 7 (1.65 g, 5.25 mmol) in CH2Cl2 were added 12.5 ml of NH3 (2 M MeOH) and the reaction was stirred at the same temperature for 1 h. The reaction mixture was concentrated, dissolved in CH2Cl2 (50 ml) and washed with sat. NaHCO3, H2O and dried over Na2SO4. Flash chromatography (SiO2, CH2Cl2/aceton 98:2) gave 9 (g, 89%) as an oil. 1H NMR (200 MHz, CDCl3): δ = 7.42 (m,5H), 6.66-6.89 (m, 3H), 5.10 (s, 2H), 4.25 (t, 2H, J = 7.06 Hz), 2.85 (t, 2H, J = 7.06 Hz), 2.05 (s, 3H), 1.27 (bs, 1H); 13C NMR (50 MHz, CDCl3): δ = 171.4, 146.1, 144.8, 136.7, 131.8, 129.1, 128.7, 128.1, 120.6, 115.6, 112.5, 71.5, 65.4, 34.8, 21.3; 10: A solution of 7 (2.8 g, 9.4 mmol) in 20 ml NH3 (2 M, MeOH) was stirred for 96 h and concentrated under reduced pressure. The residue was dissolved in CH2Cl2 (100 ml), washed with 0.1 M of citric acid (10 ml), 0.1 M NaHCO3 (10 ml) and dried over Na2SO4. The solvent was evaporated under reduced pressure to give pure 10 (2.18 g, 95%) as judged by NMR. 1H NMR (200 MHz, CDCl3): δ = 7.44-7.39 (m, 5H), 6.90-6.85 (m, 2H), 6.72-6.67 (m, 1H), 5.10 (s, 2H), 3.83 (t, 2H, J = 6.0 Hz), 2.79 (t, 2H, J = 6.0 Hz); 13C NMR (50 MHz, CDCl3): δ = 146.0, 144.5, 136.4, 132.1, 128.7, 128.3, 127.7, 120.4, 115.3, 112.4, 71.3, 63.6, 38.5.
References:
Piersanti, Giovanni;Retini, Michele;Espartero, José L.;Madrona, Andres;Zappia, Giovanni [Tetrahedron Letters,2011,vol. 52,# 38,p. 4938 - 4940] Location in patent:experimental part
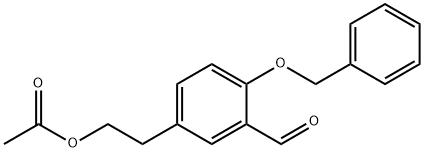
1237517-66-6
6 suppliers
$165.00/100mg

1333081-71-2
8 suppliers
$650.00/50mg
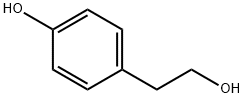
501-94-0
595 suppliers
$14.00/25g

1333081-71-2
8 suppliers
$650.00/50mg

58556-55-1
15 suppliers
$80.00/50mg

1333081-71-2
8 suppliers
$650.00/50mg
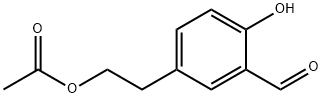
150994-00-6
6 suppliers
$165.00/250mg

1333081-71-2
8 suppliers
$650.00/50mg