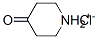
4-oxopiperidinium chloride synthesis
- Product Name:4-oxopiperidinium chloride
- CAS Number:41979-39-9
- Molecular formula:C5H10ClNO
- Molecular Weight:135.592

79099-07-3
0 suppliers
$5.00/5g
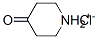
41979-39-9
0 suppliers
$15.00/10mg
Yield:41979-39-9 100%
Reaction Conditions:
with hydrogenchloride in methanol at 0 - 20;
Steps:
I.40
To a 4 N HCI/MeOH solution (100 mL) was added 1-(ferf-butyloxycarbonyl)piperidin-4-one (10 g, 50.22 mmol) at O0C, and the resulting mixture was stirred at room
References:
WO2009/76387,2009,A1 Location in patent:Page/Page column 39-40
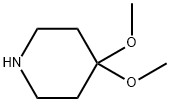
5608-82-2
11 suppliers
inquiry
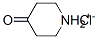
41979-39-9
0 suppliers
$15.00/10mg
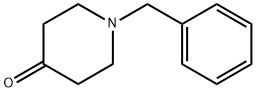
3612-20-2
445 suppliers
$6.00/10g
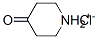
41979-39-9
0 suppliers
$15.00/10mg
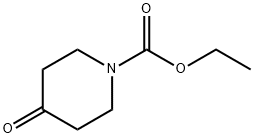
29976-53-2
1 suppliers
$5.00/5g
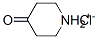
41979-39-9
0 suppliers
$15.00/10mg
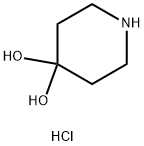
40064-34-4
0 suppliers
$10.00/5g
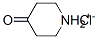
41979-39-9
0 suppliers
$15.00/10mg