4-Phenylbicyclo[2.2.2]octane-1-carboxylic acid synthesis
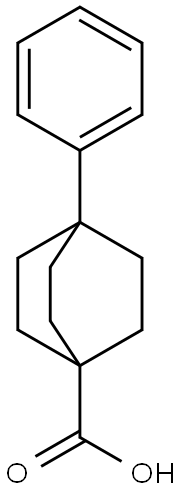
- Product Name:4-Phenylbicyclo[2.2.2]octane-1-carboxylic acid
- CAS Number:953-69-5
- Molecular formula:C15H18O2
- Molecular Weight:230.3
![Bicyclo[2.2.2]octane-1-carboxylic acid, 4-phenyl-, methyl ester](/CAS/20210111/GIF/23062-52-4.gif)
23062-52-4
1 suppliers
inquiry
![4-Phenylbicyclo[2.2.2]octane-1-carboxylic acid](/CAS/20150408/GIF/953-69-5.gif)
953-69-5
16 suppliers
inquiry
Yield:953-69-5 92%
Reaction Conditions:
Stage #1: methyl 4-phenylbicyclo<2.2.2>octane-1-carboxylatewith methanol;sodium hydroxide;water in tetrahydrofuran at 20;
Stage #2: with hydrogenchloride;water in tetrahydrofuran; pH=2;
Steps:
1.i
i) 4-Phenylbicyclo[2.2.2]octane-l-carboxylic acidTo a solution of methyl 4-phenylbicyclo[2.2.2]octane-l-carboxylate (obtained using the procedure described by N.B Chapman, S.Sotheeswaran and KJ. Toyne J. Org. Chem., 1970, 35, (4), 917) (785 mg, 3.21 mmol) in methanol (60 mL) and THF (15 niL) was added 2M NaOH (8.0 mL, 16.06 mmol). The reaction mixture was allowed to stir at ambient temperature overnight, the methanol was removed by evaporation and the residue acidified to pH 2 with 2M HCl and then extracted with EtOAc (2 x 250 mL). The organic phase was washed with brine (50 mL) separated, dried (MgSO4) and concentrated to leave a pale red solid, 682 mg (2.96 mmol, 92%);1H NMR δ 1.86 (s, 12H), 7.19 - 7.23 (m, IH), 7.31 - 7.39 (m, 4H); MS m/e MH" 229.
References:
WO2007/71966,2007,A1 Location in patent:Page/Page column 36
![Bicyclo[2.2.2]octane-1-carbonitrile, 4-phenyl-](/CAS/20210305/GIF/950-22-1.gif)
950-22-1
1 suppliers
inquiry
![4-Phenylbicyclo[2.2.2]octane-1-carboxylic acid](/CAS/20150408/GIF/953-69-5.gif)
953-69-5
16 suppliers
inquiry
![1-Bromo-4-phenylbicyclo[2.2.2]octane](/CAS/GIF/714-68-1.gif)
714-68-1
1 suppliers
inquiry
![4-Phenylbicyclo[2.2.2]octane-1-carboxylic acid](/CAS/20150408/GIF/953-69-5.gif)
953-69-5
16 suppliers
inquiry
![BICYCLO[2.2.2]OCTANE-1,4-DICARBOXYLIC ACID HEMIMETHYL ESTER](/CAS/GIF/18720-35-9.gif)
18720-35-9
245 suppliers
$19.00/1g
![4-Phenylbicyclo[2.2.2]octane-1-carboxylic acid](/CAS/20150408/GIF/953-69-5.gif)
953-69-5
16 suppliers
inquiry
![Methyl 4-broMobicyclo[2.2.2]octane-1-carboxylate](/CAS/20130318/GIF/CB72619180.gif)
23062-51-3
46 suppliers
$98.00/100mg
![4-Phenylbicyclo[2.2.2]octane-1-carboxylic acid](/CAS/20150408/GIF/953-69-5.gif)
953-69-5
16 suppliers
inquiry