
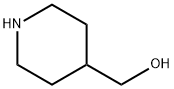
4-Piperidinemethanol, 1-butyl- synthesis
- Product Name:4-Piperidinemethanol, 1-butyl-
- CAS Number:148703-15-5
- Molecular formula:C10H21NO
- Molecular Weight:171.28
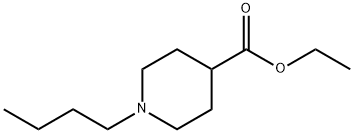
74045-89-9
13 suppliers
inquiry

148703-15-5
12 suppliers
inquiry
Yield:148703-15-5 99%
Reaction Conditions:
with lithium aluminium tetrahydride in diethyl ether at 0; for 4 h;
Steps:
1-Butyl-4-piperidinemethanol (16)
Compound 15 (6.0 g, 28 mmol) was dissolved in Et2O (20 mL) and the solution was slowly added to a cooled suspension at 0 °C of LiAlH4 (1.49 g, 39 mmol) in Et2O (20 mL). The resulting mixture was stirred at 0 °C for 4 h. Et2O was then added (40 mL) and the reaction was slowly quenched with aq 1 N NaOH under strong stirring. The precipitated residue was filtered off and rinsed with Et2O (2 ×) and the filtrate was concentrated under vacuum to afford compound 16, which was used without further purification; yield: 4.8 g (28 mmol, 99%); colorless oil. 1H NMR (CDCl3, 300 MHz): δ = 3.46 (d, J = 6.4 Hz, 2 H), 2.97-2.94 (m, 2H), 2.33-2.29 (m, 2 H), 1.95-1.89 (m, 2 H), 1.74-1.70 (m, 2 H), 1.51-1.44 (m, 3 H), 1.32-1.26 (m, 4 H), 0.89 (t, J = 7.2 Hz, 3 H). 13C NMR (CDCl3, 75 MHz): δ = 67.2, 59.0, 53.6, 38.6, 29.0, 28.7, 20.9, 14.1. HRMS (ESI+): m/z [M + H]+ calcd for C10H22NO: 172.1701; found: 172.1701. Anal. Calcd for C10H21NO: C, 70.1; H, 12.4; N, 8.2. Found: C, 69.99; H,12.29; N, 8.07.
References:
Carbonnel, Elodie;Alix, Florent;Gembus, Vincent [Synthesis,2017,vol. 49,# 9,art. no. SS-2016-T0785-OP,p. 2057 - 2062]
6457-49-4
356 suppliers
$18.00/25g

109-65-9
570 suppliers
$10.00/10g

148703-15-5
12 suppliers
inquiry
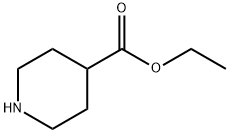
1126-09-6
470 suppliers
$15.40/25g

148703-15-5
12 suppliers
inquiry