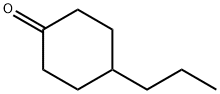
4-Propylcyclohexanone synthesis
- Product Name:4-Propylcyclohexanone
- CAS Number:40649-36-3
- Molecular formula:C9H16O
- Molecular Weight:140.22
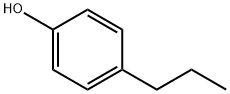
645-56-7
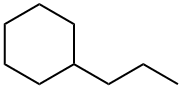
1678-92-8
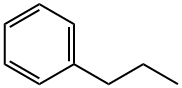
103-65-1

40649-36-3
General procedure for the synthesis of n-propylcyclohexane, n-propylbenzene, and 4-n-propylcyclohexanone from 4-propylphenol: 4-propylphenol (5.0 mmol, 681 mg), catalyst (2 wt% Pt loading, 98 mg), and water (40 mL) were added to a pre-dried high-pressure intermittent reactor (OM Lab-Tech MMJ-100, SUS316, 100 mL). After pressurization with H2 to 2 MPa at room temperature, the reactor was heated to the set reaction temperature while maintaining continuous stirring at 600 rpm. The reactor was maintained at the reaction temperature for 1 hour. Upon completion of the reaction, the reactor was cooled to room temperature and the reaction mixture was extracted with ethyl acetate, and the organic layer was analyzed by gas chromatography (GC) and gas chromatography-mass spectrometry (GC-MS) using 2-isopropylphenol as an internal standard.The GC analysis was performed using a Shimadzu GC-14B integrator (C-R8A) equipped with a capillary column (HR-1, 0.25 mm I.D. × 50 μm). GC-MS analysis was performed on a Shimadzu GC-2010/PARVUM2 equipped with the same capillary column. The catalyst was recovered by centrifugation, followed by washing with ethyl acetate and drying in an oven at 120 °C for 2 hours. The recovered catalyst can be reused for the next reaction. For time course studies, multiple batches of reactions were required at different time points. The reaction time "0" is defined as the point at which the temperature of the reaction mixture reaches the set reaction temperature.

645-56-7
201 suppliers
$11.00/1g

1678-92-8
89 suppliers
$8.00/1g

103-65-1
169 suppliers
$17.00/25mL

40649-36-3
190 suppliers
$7.00/5g
Yield:103-65-1 33% ,40649-36-3 14% ,1678-92-8 7%
Reaction Conditions:
with hydrogen in water at 300; under 15001.5 Torr; for 1 h;
Steps:
Catalytic HDO of 4-propylphenol in water
General procedure: 4-propylphenol (5.0 mmol, 681 mg), cata-lyst (2 wt% Pt loading, 98 mg) and water (40 mL) were charged ina well dried high-pressure batch reactor (OM Lab-Tech MMJ-100,SUS316, 100 mL). After pressurization with H2to 2 MPa at roomtemperature, the reactor was heated to the desired reaction tem-perature with continuous stirring at 600 rpm. Then, the reactor waskept at the reaction temperature for 1 h. After cooling to room tem-perature, the reaction mixture was extracted with ethyl acetateand the organic layer was analyzed by GC and GC-MS using 2-isopropylphenol as an internal standard. GC analyses were carriedout using a Shimadzu GC-14B equipped with an integrator (C-R8A)with a capillary column (HR-1, 0.25 mm i.d. × 50 m). GC-MS anal-yses were measured by a Shimadzu GC-2010/PARVUM2 equippedwith the same column. The catalyst was recovered by centrifuga-tion, followed by washing with ethyl acetate and dried in an ovenat 120C for 2 h. The recovered catalyst was reused in the nextreaction.For the time-course study, many batch reactions at differenttime were performed separately. Here, the reaction time “0” wasdefined as the time when the temperature of the reaction mixturejust reached the reaction temperature.
References:
Ohta, Hidetoshi;Feng, Bo;Kobayashi, Hirokazu;Hara, Kenji;Fukuoka, Atsushi [Catalysis Today,2014,vol. 234,p. 139 - 144]

645-56-7
201 suppliers
$11.00/1g

40649-36-3
190 suppliers
$7.00/5g
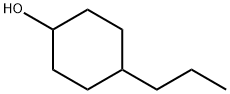
52204-65-6
89 suppliers
$45.00/5g

52204-65-6
89 suppliers
$45.00/5g

40649-36-3
190 suppliers
$7.00/5g

645-56-7
201 suppliers
$11.00/1g

40649-36-3
190 suppliers
$7.00/5g

645-56-7
201 suppliers
$11.00/1g

40649-36-3
190 suppliers
$7.00/5g
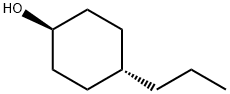
77866-58-1
56 suppliers
$287.00/1g
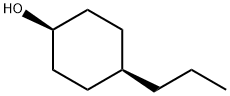
98790-22-8
3 suppliers
inquiry