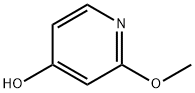
4-Pyridinol,2-methoxy-(6CI,9CI) synthesis
- Product Name:4-Pyridinol,2-methoxy-(6CI,9CI)
- CAS Number:66080-45-3
- Molecular formula:C6H7NO2
- Molecular Weight:125.13
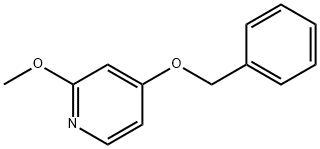
66080-44-2

66080-45-3
General procedure for the synthesis of 2-methoxy-4-pyridinol from 4-(benzyloxy)-2-methoxypyridine: In Example (16a), prepared 4-(benzyloxy)-2-methoxypyridine (4.65 g, 21.6 mmol) and 10% palladium carbon (2.30 g, 2.16 mmol) were dissolved in ethanol (100 mL). The mixture was stirred at room temperature for 30 minutes under hydrogen atmosphere. Upon completion of the reaction, the insoluble material was removed by filtration and the filtrate was subsequently concentrated to afford 2-methoxy-4-pyridinol (2.70 g, 99%) as a colorless oily product. Its 1H-NMR (400 MHz, CDCl3) data were as follows: δppm: 3.90 (3H, s), 6.13 (1H, s), 6.42 (1H, d, J = 5.9 Hz), 7.80 (1H, brs).

66080-44-2
12 suppliers
inquiry

66080-45-3
95 suppliers
$20.00/1g
Yield: 99%
Reaction Conditions:
with hydrogen;5%-palladium/activated carbon in ethanol at 20; for 2 h;
Steps:
16.16.b
(16b) 2-Methoxypyridin-4-ol A solution of 4-(benzyloxy)-2-methoxypyridine produced in Example (16a) (4.65 g, 21.6 mmol) and 10% palladium carbon (2.30 g, 2.16 mmol) in ethanol (100 mL) was stirred in a hydrogen atmosphere at room temperature for 30 minutes. The insoluble matter was separated by filtration and then the filtrate was concentrated to obtain the title compound (2.70 g, 99%) as a colorless oil. 1H-NMR (400 MHz, CDCl3): δ ppm: 3.90 (3H, s), 6.13 (1H, s), 6.42 (1H, d, J = 5.9 Hz), 7.80 (1H, brs).
References:
Daiichi Sankyo Company, Limited EP2404918, 2012, A1 Location in patent:Page/Page column 34
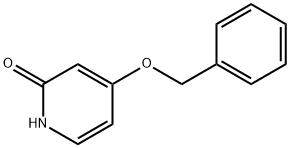
53937-02-3
167 suppliers
$60.00/5 g

66080-45-3
95 suppliers
$20.00/1g

53937-02-3
167 suppliers
$60.00/5 g

66080-45-3
95 suppliers
$20.00/1g