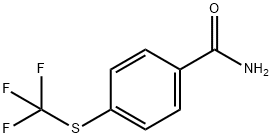
4-(TRIFLUOROMETHYLTHIO)BENZAMIDE 97 synthesis
- Product Name:4-(TRIFLUOROMETHYLTHIO)BENZAMIDE 97
- CAS Number:330-15-4
- Molecular formula:C8H6F3NOS
- Molecular Weight:221.2
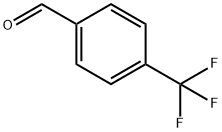
455-19-6
414 suppliers
$6.00/5g

330-15-4
16 suppliers
$31.80/1gm:
Yield:330-15-4 71%
Reaction Conditions:
with nitromethane;trifluoromethylsulfonic anhydride;acetic acid in formic acid at 80 - 120;
Steps:
61 Example 61 p-Trifluoromethylbenzamide
Take a reaction tube and add 60-100mg (1.2mmol) of nitromethane, 40-60mg (0.3mmol) of p-trifluoromethylbenzaldehyde, 0.5mL of acetic acid, 150-200mg (0.6mmol) of trifluoromethanesulfonic anhydride, and formic acid 30-60mg (0.75mmol), stirred at 80-120°C for 1-72 hours. After the completion of the reaction, 10 mL of sodium hydroxide solution was added to quench the reaction, extracted with ethyl acetate 3 times, the organic phase was washed with 5 mL of brine, and the organic phases were combined and separated by column chromatography to obtain 40.3 mg of p-trifluoromethylbenzamide. The yield was 71%.
References:
CN112574054,2021,A Location in patent:Paragraph 0305-0308
5030-59-1
0 suppliers
inquiry

330-15-4
16 suppliers
$31.80/1gm: