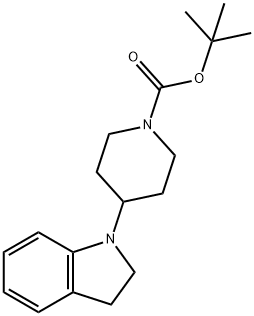
tert-butyl 4-(indolin-1-yl)piperidine-1-carboxylate synthesis
- Product Name:tert-butyl 4-(indolin-1-yl)piperidine-1-carboxylate
- CAS Number:400828-91-3
- Molecular formula:C18H26N2O2
- Molecular Weight:302.41
496-15-1
436 suppliers
$8.00/10g

79099-07-3
0 suppliers
$5.00/5g

400828-91-3
17 suppliers
$205.00/1g
Yield:400828-91-3 100%
Reaction Conditions:
with sodium tris(acetoxy)borohydride;acetic acid in 1,2-dichloro-ethane at 0 - 65; for 65 h;Product distribution / selectivity;
Steps:
Intermediate 1; 1 ,1 -Dimethylethyl 4-(2,3-dihydro-1 H-indol-1 -yl)-1 -piperidinecarboxylateTo a solution of indoline (Acros, 1Og, 83.91 mmol) in 1 ,2-DCE (20OmL) under argon, cooled in ice was added 1-Boc-4-piperidinone (Fluka, 16.72g, 83.91 mmol) and acetic acid (4.8ml_, 83.91 mmol). After stirring for 10 minutes sodium triacetoxyborohydride (26.68g, 125.87mmol) was added portionwise, keeping the temperature below 100C. The flask was then removed from the ice bath and stirring under argon at room temperature was continued for 65 hours. The solvent was removed in vacuo and the residue was taken up in ethyl acetate (40OmL) and washed with saturated aqueous sodium bicarbonate solution (3x10OmL). The ethyl acetate layer was dried over sodium sulfate, filtered and concentrated in vacuo to afford the title compound as a pale brown oil (27.52g, 100%).1H NMR (CDCI3, 400MHz): δ 1.47 (9H, s), 1.50-1.68 (4H, m, integration slightly obscured by adjacent peak), 1.74-1.86 (2H, m), 2.78 (2H, br.m), 2.94 (2H, t, J=8.5), 3.34 (2H, t, J=8.5), 3.51 (1 H, tt, J=1 1.5, 3.5), 6.42 (1 H, d, J=7.5), 6.61 (1 H, dd, J=7.5, 1.0) 7.01-7.09 (2H, m). Mass spectrum (ESI): Ci8H26N2O2 requires 302; found 303 (MH+).
References:
WO2009/16225,2009,A1 Location in patent:Page/Page column 14