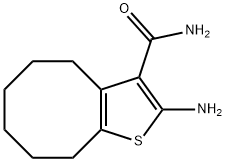
2-AMINO-4,5,6,7,8,9-HEXAHYDROCYCLOOCTA[B]THIOPHENE-3-CARBOXAMIDE synthesis
- Product Name:2-AMINO-4,5,6,7,8,9-HEXAHYDROCYCLOOCTA[B]THIOPHENE-3-CARBOXAMIDE
- CAS Number:40106-15-8
- Molecular formula:C11H16N2OS
- Molecular Weight:224.32
![2-amino-4,5,6,7,8,9-hexahydrocycloocta[b]thiophene-3-carbonitrile(SALTDATA: FREE)](/CAS/GIF/40106-14-7.gif)
40106-14-7
24 suppliers
inquiry
![2-AMINO-4,5,6,7,8,9-HEXAHYDROCYCLOOCTA[B]THIOPHENE-3-CARBOXAMIDE](/CAS/GIF/40106-15-8.gif)
40106-15-8
25 suppliers
$45.00/50mg
Yield:40106-15-8 109 mg
Reaction Conditions:
with sulfuric acid
Steps:
2-amino-4,5,6,7,8,9-hexahydrocycloocta[b]thiophene-3-carboxamide (7).
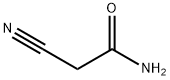
Cyclooctanone 1d (0.5 mL, 3.80 mmol), malononitrile (276 mg, 4.18 mmol) and elemental sulphur (134 mg, 4.18 mmol) were suspended in DMF (1 mL) and to this mixture was added Et3N (1 mL) and stirred at room temperature overnight. The mixture was diluted with EtOAc (30 mL) and washed with 0.5 M HCl (15 mL), then water (3 x 15 mL) and finally brine (10 mL). The organic layer was dried (MgSO4), filtered and then concentrated to a residue. The residue was chromatographed on silica gel eluting with 30% EtOAc-pet spirits providing 6 as a yellow solid that was used directly in the next step. The nitrile 6 (340mg, 1.65 mmol) was suspended in neat H2SO4 (2 mL) and vigorously stirred overnight. The resulting dark brown solution was quenched with crushed ice and neutralised with solid Na2CO3 and the extracted with EtOAc (3 x 20 mL). The combined organics were washed with saturated bicarbonate solution (30 mL), then water and finally brine (10 mL). The organic layer was dried (MgSO4), filtered and then concentrated affording 7 as a solid that was recrystallised from MeOH.
References:
Aurelio, Luigi;Christopoulos, Arthur;Flynn, Bernard L.;Scammells, Peter J.;Sexton, Patrick M.;Valant, Celine [Bioorganic and Medicinal Chemistry Letters,2011,vol. 21,# 12,p. 3704 - 3707] Location in patent:supporting information; experimental part
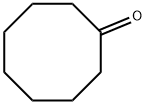
502-49-8
290 suppliers
$8.00/5g
107-91-5
568 suppliers
$5.00/10g
![2-AMINO-4,5,6,7,8,9-HEXAHYDROCYCLOOCTA[B]THIOPHENE-3-CARBOXAMIDE](/CAS/GIF/40106-15-8.gif)
40106-15-8
25 suppliers
$45.00/50mg

502-49-8
290 suppliers
$8.00/5g
![2-AMINO-4,5,6,7,8,9-HEXAHYDROCYCLOOCTA[B]THIOPHENE-3-CARBOXAMIDE](/CAS/GIF/40106-15-8.gif)
40106-15-8
25 suppliers
$45.00/50mg

40106-06-7
1 suppliers
inquiry
![2-AMINO-4,5,6,7,8,9-HEXAHYDROCYCLOOCTA[B]THIOPHENE-3-CARBOXAMIDE](/CAS/GIF/40106-15-8.gif)
40106-15-8
25 suppliers
$45.00/50mg