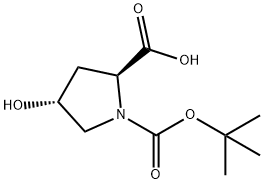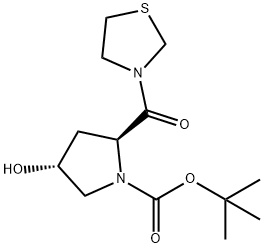
(2S,4R)-4-Hydroxy-2-(3-thiazolidinylcarbonyl)-1-pyrrolidinecarboxylic acid tert-butyl ester synthesis
- Product Name:(2S,4R)-4-Hydroxy-2-(3-thiazolidinylcarbonyl)-1-pyrrolidinecarboxylic acid tert-butyl ester
- CAS Number:401564-30-5
- Molecular formula:C13H22N2O4S
- Molecular Weight:302.39
Yield: 62%
Reaction Conditions:
with benzotriazol-1-ol;1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride in N,N-dimethyl-formamide at 20; for 18 h;
Steps:
5.1.2. 3-((S)-1-tert-Butoxycarbonyl-4-oxopyrrolidin-2-ylcarbonyl)thiazolidine (7a)
A mixture of N-tert-butoxycarbonyl-l-trans-hydroxyproline (6a) (69.4 g, 0.3 mol), thiazolidine (29.4 g, 0.33 mmol), HOBT (50.5 g, 0.33 mol) and EDC (63.3 g, 0.33 mol) in DMF (300 mL) was stirred at room temperature for 18 h. The reaction mixture was concentrated under reduced pressure. To the residue was added a saturated aqueous sodium hydrogen carbonate solution and the mixture was extracted with ethyl acetate. The extract was dried and concentrated under reduced pressure to give 3-[(2S,4R)-1-tert-butoxycarbonyl-4-hydroxypyrrolidin-2-ylcarbonyl]thiazolidine (56.3 g, 62%) as a colorless oil. 1H NMR (300 MHz, CDCl3): δ 1.41-1.45 (9H, m), 1.95-2.34 (2H, m), 2.62-3.25 (2H, m), 3.40-3.98 (4H, m), 4.40-4.90 (4H, m).To a solution of the above compound (55.4 g, 183 mmol) and triethylamine (46 mL, 330 mmol) in dichloromethane (350 mL) was added sulfur trioxide-pyridine complex (52.4 g, 329 mmol) in DMSO (150 mL) under ice cooling and the mixture was stirred for 2 h. The reaction mixture was poured into a saturated aqueous sodium hydrogen carbonate solution and extracted with ethyl acetate. The extract was washed with brine, dried and concentrated under reduced pressure. The residue purified by silica gel chromatography with n-hexane/ethyl acetate (1:1, v/v) to give the title compound (30.3 g, 55%) as a white powder.
References:
Yoshida, Tomohiro;Akahoshi, Fumihiko;Sakashita, Hiroshi;Sonda, Shuji;Takeuchi, Masahiro;Tanaka, Yoshihito;Nabeno, Mika;Kishida, Hiroyuki;Miyaguchi, Ikuko;Hayashi, Yoshiharu [Bioorganic and Medicinal Chemistry,2012,vol. 20,# 16,p. 5033 - 5041] Location in patent:experimental part