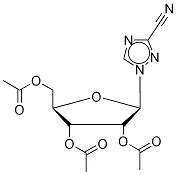
3-Cyano-1-(2,3,5-tri-O-acetyl-β-D-ribofuranosyl)-1,2,4-triazole synthesis
- Product Name:3-Cyano-1-(2,3,5-tri-O-acetyl-β-D-ribofuranosyl)-1,2,4-triazole
- CAS Number:40371-99-1
- Molecular formula:C14H16N4O7
- Molecular Weight:352.3
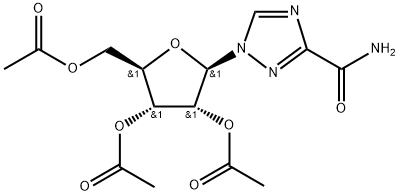
40372-03-0
9 suppliers
inquiry

40371-99-1
7 suppliers
inquiry
Yield:40371-99-1 87%
Reaction Conditions:
with triethylamine;trichlorophosphate in chloroform at 0 - 20; for 17 h;Concentration;
Steps:
1D Synthesis of 3-cyano-2’,3’,5’-O-triacetyl-3-D-ribo- furanosyl)-1,2,4-triazole 9
Synthesis of 3-cyano-2',3',5'-O-triacetyl-β-D-ribofuranosyl)-1,2,4-triazole 9 A mixture of crude 2’,3’,5’-O-triacetyl-fi-D-ribofurano- syl)-1,2,4-triazole-3-carboxamide (compound 8) (934 mg) and triethylamine (5.5 mE) in chloroform (10 mE) was cooled to 0° C. in an ice-water bath. Phosphorus oxychloride (0.7 mE, 7.57 mmol) was added dropwise with stirring and the solution was allowed to warm to room temperature. Afier the mixture was stirred at room temperature for 17 hours, the brown reaction mixture was concentrated to dryness in vacuum and the residue was dissolved in dichloromethane (20 mE). The organic solution was washed with saturated aqueous sodium bicarbonate (3x60 mE), dried over sodium sulfate, and concentrated. The residue was purified by columnchromatography on silica gel using MeOH/DCM (2%) toafford the product (0.7 18 g, 87% yield based on ribavirin).
References:
US9149538,2015,B2 Location in patent:Page/Page column 36; 37
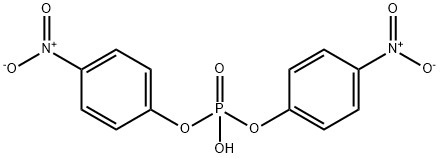
645-15-8
165 suppliers
$26.00/1g
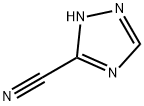
3641-10-9
127 suppliers
$10.00/1g
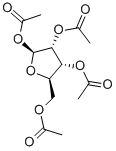
13035-61-5
546 suppliers
$5.00/25g

40371-99-1
7 suppliers
inquiry
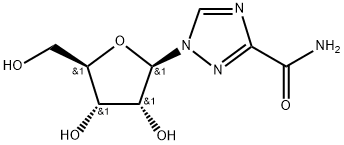
36791-04-5
564 suppliers
$5.00/100mg

40371-99-1
7 suppliers
inquiry