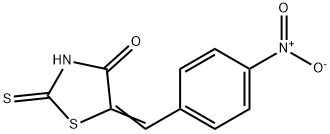
5-[1-(4-NITRO-PHENYL)-METH-(Z)-YLIDENE]-2-THIOXO-THIAZOLIDIN-4-ONE synthesis
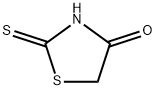
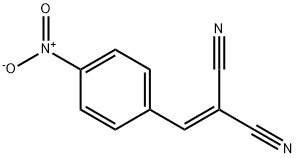
- Product Name:5-[1-(4-NITRO-PHENYL)-METH-(Z)-YLIDENE]-2-THIOXO-THIAZOLIDIN-4-ONE
- CAS Number:4120-64-3
- Molecular formula:C10H6N2O3S2
- Molecular Weight:266.3
Yield:4120-64-3 98%
Reaction Conditions:
with sodium acetate;acetic acid at 120;
Steps:
4.1.6 5-Arylidenerhodanine compounds 5(A-G)
General procedure: A mixture of rhodanine (3mmol), different aldehydes (3mmol) and anhydrous sodium acetate (3mmol) were taken in glacial acetic acid (10mL). The reaction mixture was heated to 120°C in oil bath for 3-4h. The reaction was monitored by TLC (n-hexane/ AcOEt=3:2). Upon completion, the reaction mixture was cooled and the formed precipitate was filtered, washed with water and recrystallized from methanol to yield compounds (5A-5G) [9,26-28].
References:
Salem, Manar G.;Abdel Aziz, Yasmine M.;Elewa, Marwa;Nafie, Mohamed S.;Elshihawy, Hosam A.;Said, Mohamed M. [Bioorganic Chemistry,2021,vol. 111,art. no. 104909]