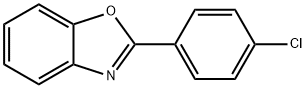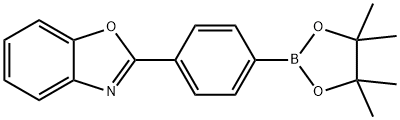
2-(4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)benzo[d]oxazole synthesis
- Product Name:2-(4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)benzo[d]oxazole
- CAS Number:439090-73-0
- Molecular formula:C19H20BNO3
- Molecular Weight:321.18
![2-(4-bromophenyl)benzo[d]oxazole](/CAS/GIF/3164-13-4.gif)
3164-13-4

73183-34-3
![2-(4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)benzo[d]oxazole](/CAS/GIF/439090-73-0.gif)
439090-73-0
2-(4-Bromophenyl)benzo[d]oxazole (5 g, 18.2 mmol), pinacol ester of bisboronic acid (5.1 g, 20.1 mmol) and potassium acetate (5.4 g, 54.7 mmol) were dissolved in 1,4-dioxane (182 ml) and stirred to mix. Subsequently, Pd(dppf)Cl2 (260 mg, 0.36 mmol) was added, and the reaction mixture was heated to 100 °C and stirred for 8 hours. After completion of the reaction, the reaction solution was cooled to room temperature. Water (100 ml) was added to the reaction solution and stirred for 10 min before extraction with tetrahydrofuran (THF). The organic layer was separated and the aqueous layer was discarded. The organic layer was dried with anhydrous magnesium sulfate (MgSO4) and subsequently concentrated. The concentrate was recrystallized with ethanol (150 ml) and filtered to give the target compound 2-(4-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)benzo[d]oxazole (5.3 g, 90% yield). Mass spectrometry result: [M + H] = 322.
![2-(4-bromophenyl)benzo[d]oxazole](/CAS/GIF/3164-13-4.gif)
3164-13-4
155 suppliers
$16.00/250mg

73183-34-3
587 suppliers
$6.00/5g
![2-(4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)benzo[d]oxazole](/CAS/GIF/439090-73-0.gif)
439090-73-0
31 suppliers
inquiry
Yield:439090-73-0 99%
Reaction Conditions:
with (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride;potassium acetate in toluene; for 4 h;Reflux;Inert atmosphere;
Steps:
7.19 Step 19 (Synthesis of 2-(benzo[d]oxazol-2-yl)phenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane)
2-(4-Bromophenyl)benzo[d]azole (10 parts), bis(pinacol)diboron (10.8 parts), potassium acetate (6.9 parts) and [1,1'-double ( Diphenylphosphino)ferrocene]palladium(II) chloride dichloride adduct (1.0 part) was mixed in toluene (500 parts), and stirred at reflux temperature for 4 hours under a nitrogen atmosphere.After the obtained reaction liquid was cooled to room temperature, 20 parts of tannin was added, and the mixture was stirred for 5 minutes.Then, by separating the solid fraction by filtration, and removing the solvent under reduced pressure,2-(Benzo[d]oxazol-2-yl)phenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane pentane (11.4 parts, yield 99%).
References:
TW2018/31491,2018,A Location in patent:Paragraph 0090
![1 -phenyl-2-(4-(4,4,5,5-tetramethyl- 1 ,3,2-dioxaborolan-2-yl)phenyl)-1H-benzo[d]imidazole](/CAS/20180527/GIF/1146340-38-6.gif)
1146340-38-6
32 suppliers
inquiry

73183-34-3
587 suppliers
$6.00/5g
![2-(4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)benzo[d]oxazole](/CAS/GIF/439090-73-0.gif)
439090-73-0
31 suppliers
inquiry
![2-(4-bromophenyl)benzo[d]oxazole](/CAS/GIF/3164-13-4.gif)
3164-13-4
155 suppliers
$16.00/250mg

73183-34-3
587 suppliers
$6.00/5g
357437-74-2
55 suppliers
$44.00/250mg
![2-(4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)benzo[d]oxazole](/CAS/GIF/439090-73-0.gif)
439090-73-0
31 suppliers
inquiry
586-75-4
227 suppliers
$10.00/5g
![2-(4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)benzo[d]oxazole](/CAS/GIF/439090-73-0.gif)
439090-73-0
31 suppliers
inquiry