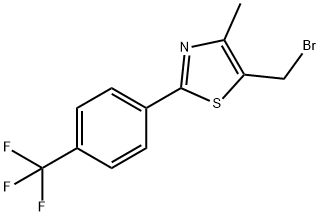
5-(BROMOMETHYL)-4-METHYL-2-[4-(TRIFLUOROMETHYL)PHENYL]-1,3-THIAZOLE synthesis
- Product Name:5-(BROMOMETHYL)-4-METHYL-2-[4-(TRIFLUOROMETHYL)PHENYL]-1,3-THIAZOLE
- CAS Number:439134-78-8
- Molecular formula:C12H9BrF3NS
- Molecular Weight:336.17
![(4-METHYL-2-[4-(TRIFLUOROMETHYL)PHENYL]-1,3-THIAZOL-5-YL)METHANOL](/CAS/GIF/317318-96-0.gif)
317318-96-0
![5-(BROMOMETHYL)-4-METHYL-2-[4-(TRIFLUOROMETHYL)PHENYL]-1,3-THIAZOLE](/CAS/GIF/439134-78-8.gif)
439134-78-8
GENERAL STEPS: (4-methyl-2-(4-(trifluoromethyl)phenyl)thiazol-5-yl)methanol (15.0 g, 55.0 mmol) obtained in Example 2 was dissolved in anhydrous dichloromethane (300 mL). Triphenylphosphine (TPP, 5.7 g, 60.0 mmol, 1.1 eq.) and tetrabromomethane (20.0 g, 60.0 mmol, 1.1 eq.) were added sequentially to the mixture at room temperature. After 1 h of reaction, the solvent was removed by evaporation under reduced pressure. The residual triphenylphosphine oxide was precipitated with a mixture of hexane and ethyl acetate (v/v = 5/1), filtered and evaporated under reduced pressure to afford 5-(bromomethyl)-4-methyl-2-[4-(trifluoromethyl)phenyl]-1,3-thiazole (17.2 g, yield: 93%).1H-NMR (300 MHz, CDCl3): δ 8.00 (d, 2H, J = 8.1 Hz), 7.67 (d, 2H, J = 8.2 Hz), 4.72 (s, 2H), 2.47 (s, 3H).13C-NMR (78.5 MHz, CDCl3): δ 165.0, 153.8, 136.9, 132.4, 129.7 (q), 127.0, 126.3 (m), 122.5, 23.8. 15.5.
![(4-METHYL-2-[4-(TRIFLUOROMETHYL)PHENYL]-1,3-THIAZOL-5-YL)METHANOL](/CAS/GIF/317318-96-0.gif)
317318-96-0
70 suppliers
$65.00/50mg
![5-(BROMOMETHYL)-4-METHYL-2-[4-(TRIFLUOROMETHYL)PHENYL]-1,3-THIAZOLE](/CAS/GIF/439134-78-8.gif)
439134-78-8
43 suppliers
$72.50/250mg
Yield:439134-78-8 93%
Reaction Conditions:
with carbon tetrabromide;triphenylphosphine in dichloromethane at 20; for 1 h;
Steps:
3 Example 3; Preparation [OF 5-BROMOMETHYL-4-METHYL-2- [ (4-TRIFLUOROMETHYL)] phenyl] thiazole [Step C]
[[4-METHYL-2- (4-TRIFLUOROMETHYL-PHENYL) THIAZOLE-5-YL]] methanol (15.0 g, 55.0 mmol) obtained from Example 2 was dissolved in anhydrous dichloromethane [(300] [MNo. ], and then triphenylphosphine (TPP, 5. 7 g, 60.0 mmol, 1.1 eq. ) and tetrabromomethane (20.0 [G,] 60.0 mmol, 1.1 eq. ) were added to the mixture [SEQUENCIALLY] at room temperature. After 1 hour, the solvent was evaporated from the reaction mixture under reduced pressure. Subsequently, the remained triphenylphosphine oxide was precipitated by a mixed solvent of hexane and ethyl acetate (v/v = [5/1),] followed by filtration and evaporation under reduced pressure to thereby yield [17. 2] g of the title compound (yield: 93%). 'H-NMR (300 MHz, [CDC13)] : 8.00 (d, 2H, [J =] 8.1 Hz), 7.67 (d, 2H, [J =] 8.2 Hz), 4.72 (s, 2H), 2.47 (s, 3H). [13C-NMR] (78. [5] MHz, [CDC13)] : 165. 0, [153.] 8, [136. 9, 132. 4,] 129.7 (q), [127.] 0, 126.3 (m), 122.5, 23.8, 15.5.
References:
WO2003/106442,2003,A1 Location in patent:Page 13
72505-21-6
166 suppliers
$10.00/5g
![5-(BROMOMETHYL)-4-METHYL-2-[4-(TRIFLUOROMETHYL)PHENYL]-1,3-THIAZOLE](/CAS/GIF/439134-78-8.gif)
439134-78-8
43 suppliers
$72.50/250mg
![Ethyl 4-methyl-2-[4-(trifluoromethyl)phenyl]-1,3-thiazole-5-carboxylate](/CAS/GIF/175277-03-9.gif)
175277-03-9
123 suppliers
$15.00/5g
![5-(BROMOMETHYL)-4-METHYL-2-[4-(TRIFLUOROMETHYL)PHENYL]-1,3-THIAZOLE](/CAS/GIF/439134-78-8.gif)
439134-78-8
43 suppliers
$72.50/250mg