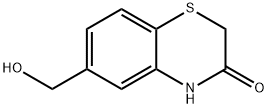
6-(HydroxyMethyl)-2h-benzo[b][1,4]thiazin-3(4h)-one synthesis
- Product Name:6-(HydroxyMethyl)-2h-benzo[b][1,4]thiazin-3(4h)-one
- CAS Number:443955-31-5
- Molecular formula:C9H9NO2S
- Molecular Weight:195.24
![3,4-Dihydro-3-oxo-2H-benzo[b][1,4]thiazine-6-carboxylic acid](/CAS/GIF/272437-84-0.gif)
272437-84-0
56 suppliers
$45.00/50mg
![6-(HydroxyMethyl)-2h-benzo[b][1,4]thiazin-3(4h)-one](/CAS/GIF/443955-31-5.gif)
443955-31-5
15 suppliers
inquiry
Yield:443955-31-5 89%
Reaction Conditions:
Stage #1: 3-oxo-3,4-dihydro-2H-benzo[b][1,4]thiazine-6-carboxylic acidwith triethylamine;isobutyl chloroformate in tetrahydrofuran at 0; for 2 h;
Stage #2: with sodium tetrahydroborate in tetrahydrofuran;water at 0 - 20; for 18 h;
Stage #3: with hydrogenchloride in tetrahydrofuran;water;
Steps:
H.B 3-Oxo-3,4-dihydro-2H-benzo[1,4]thiazine-6-carbaldehyde
B. 6-Hydroxymethyl-4H-benzo[1,4]thiazin-3-one. To a 0° C. suspension of 3-oxo-3,4-dihydro-2H-benzo[1,4]thiazine-6-carboxylic acid (400 mg, 2 mmol) in THF (19 mL) was added Et3N (0.32 mL, 2 mmol) and the resulting solution was treated with iso-butylchloroformate (0.3 mL, 2 mmol). The resulting suspension was stirred at 0° C. for 2 h, filtered and the solid was washed with THF. The filtrate was cooled to 0° C., treated with NaBH4 (150 mg, 4.3 mmol) and slowly treated with H2O (10 mL). The resulting suspension was, warmed to RT and stirred for 18 h. The suspension was neutralized using 1 N HCl and diluted with H2O (100 mL). The organic layer was extracted with EtOAc (3*200 mL). The combined organic layers were dried (MgSO4), filtered and concentrated. The resulting residue was purified on SiO2 (0-10% CH3OH/CH2Cl2) to provide 330 mg (89%) of the title compound as a white solid. MS (ESI): exact mass calculated for C9H9NO2S, 195.04; m/z found, 196.1 [M+H]+. 1H NMR (500 MHz, CD3OD): 7.26 (d, J=7.9, 1H), 7.01-6.98 (m, 2H), 4.55 (s, 2H), 3.40 (s, 2H).
References:
US2006/223810,2006,A1 Location in patent:Page/Page column 22